Input interpretation

praseodymium(III) nitrate, hexahydrate | elemental composition
Result
Find the elemental composition for praseodymium(III) nitrate, hexahydrate in terms of the atom and mass percents: atom percent = N_i/N_atoms × 100% mass percent = (N_im_i)/m × 100% Plan: • Write the chemical formula and gather atomic masses from the periodic table. • Determine values for N_i, m_i, N_atoms and m using these items. • Finally, compute the percents and check the results. Write the chemical formula: Pr(NO_3)_3·6H_2O Use the chemical formula, Pr(NO_3)_3·6H_2O, to count the number of atoms, N_i, for each element and find the total number of atoms, N_atoms: | number of atoms H (hydrogen) | 2 N (nitrogen) | 3 O (oxygen) | 10 Pr (praseodymium) | 2 N_atoms = 2 + 3 + 10 + 2 = 17 Divide each N_i by N_atoms to calculate atom fractions. Then use the property that atom fractions must sum to one to check the work: | number of atoms | atom fraction H (hydrogen) | 2 | 2/17 N (nitrogen) | 3 | 3/17 O (oxygen) | 10 | 10/17 Pr (praseodymium) | 2 | 2/17 Check: 2/17 + 3/17 + 10/17 + 2/17 = 1 Compute atom percents using the atom fractions: | number of atoms | atom percent H (hydrogen) | 2 | 2/17 × 100% = 11.8% N (nitrogen) | 3 | 3/17 × 100% = 17.6% O (oxygen) | 10 | 10/17 × 100% = 58.8% Pr (praseodymium) | 2 | 2/17 × 100% = 11.8% Look up the atomic mass, m_i, in unified atomic mass units, u, for each element in the periodic table: | number of atoms | atom percent | atomic mass/u H (hydrogen) | 2 | 11.8% | 1.008 N (nitrogen) | 3 | 17.6% | 14.007 O (oxygen) | 10 | 58.8% | 15.999 Pr (praseodymium) | 2 | 11.8% | 140.90766 Multiply N_i by m_i to compute the mass for each element. Then sum those values to compute the molecular mass, m: | number of atoms | atom percent | atomic mass/u | mass/u H (hydrogen) | 2 | 11.8% | 1.008 | 2 × 1.008 = 2.016 N (nitrogen) | 3 | 17.6% | 14.007 | 3 × 14.007 = 42.021 O (oxygen) | 10 | 58.8% | 15.999 | 10 × 15.999 = 159.990 Pr (praseodymium) | 2 | 11.8% | 140.90766 | 2 × 140.90766 = 281.81532 m = 2.016 u + 42.021 u + 159.990 u + 281.81532 u = 485.84232 u Divide the mass for each element by m to calculate mass fractions. Then use the property that mass fractions must sum to one to check the work: | number of atoms | atom percent | mass fraction H (hydrogen) | 2 | 11.8% | 2.016/485.84232 N (nitrogen) | 3 | 17.6% | 42.021/485.84232 O (oxygen) | 10 | 58.8% | 159.990/485.84232 Pr (praseodymium) | 2 | 11.8% | 281.81532/485.84232 Check: 2.016/485.84232 + 42.021/485.84232 + 159.990/485.84232 + 281.81532/485.84232 = 1 Compute mass percents using the mass fractions: Answer: | | | number of atoms | atom percent | mass percent H (hydrogen) | 2 | 11.8% | 2.016/485.84232 × 100% = 0.4149% N (nitrogen) | 3 | 17.6% | 42.021/485.84232 × 100% = 8.649% O (oxygen) | 10 | 58.8% | 159.990/485.84232 × 100% = 32.93% Pr (praseodymium) | 2 | 11.8% | 281.81532/485.84232 × 100% = 58.01%
Mass fraction pie chart
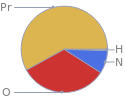
Mass fraction pie chart