Input interpretation
sodium bromide | potassium chloride
Chemical names and formulas
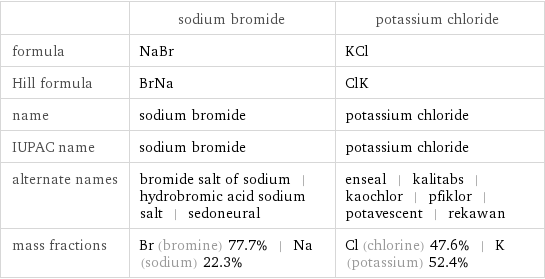
| sodium bromide | potassium chloride formula | NaBr | KCl Hill formula | BrNa | ClK name | sodium bromide | potassium chloride IUPAC name | sodium bromide | potassium chloride alternate names | bromide salt of sodium | hydrobromic acid sodium salt | sedoneural | enseal | kalitabs | kaochlor | pfiklor | potavescent | rekawan mass fractions | Br (bromine) 77.7% | Na (sodium) 22.3% | Cl (chlorine) 47.6% | K (potassium) 52.4%
Structure diagrams

Structure diagrams
Basic properties
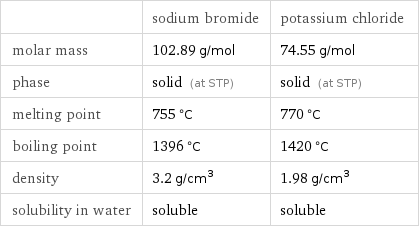
| sodium bromide | potassium chloride molar mass | 102.89 g/mol | 74.55 g/mol phase | solid (at STP) | solid (at STP) melting point | 755 °C | 770 °C boiling point | 1396 °C | 1420 °C density | 3.2 g/cm^3 | 1.98 g/cm^3 solubility in water | soluble | soluble
Units
