Input interpretation
bromide 2 propylzinc bromide
Basic properties
![molar mass | 268.3 g/mol formula | C_3H_7Br_2Zn empirical formula | Br_2C_3Zn_H_7 SMILES identifier | CC(C)[Zn+2].[Br-].[Br-] InChI identifier | InChI=1/C3H7.2BrH.Zn/c1-3-2;;;/h3H, 1-2H3;2*1H;/q;;;+2/p-2/fC3H7.2Br.Zn/h;2*1h;/q;2*-1;m InChI key | AZOKBKFZEJDIAU-UHFFFAOYSA-L](../image_source/6e66d6c0db127deeb9f7dc3acf432c36.png)
molar mass | 268.3 g/mol formula | C_3H_7Br_2Zn empirical formula | Br_2C_3Zn_H_7 SMILES identifier | CC(C)[Zn+2].[Br-].[Br-] InChI identifier | InChI=1/C3H7.2BrH.Zn/c1-3-2;;;/h3H, 1-2H3;2*1H;/q;;;+2/p-2/fC3H7.2Br.Zn/h;2*1h;/q;2*-1;m InChI key | AZOKBKFZEJDIAU-UHFFFAOYSA-L
Structure diagram
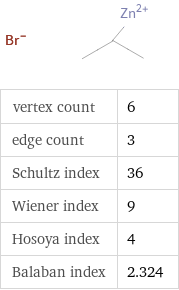
vertex count | 6 edge count | 3 Schultz index | 36 Wiener index | 9 Hosoya index | 4 Balaban index | 2.324
Quantitative molecular descriptors
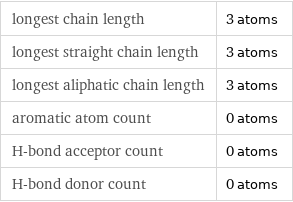
longest chain length | 3 atoms longest straight chain length | 3 atoms longest aliphatic chain length | 3 atoms aromatic atom count | 0 atoms H-bond acceptor count | 0 atoms H-bond donor count | 0 atoms
Elemental composition
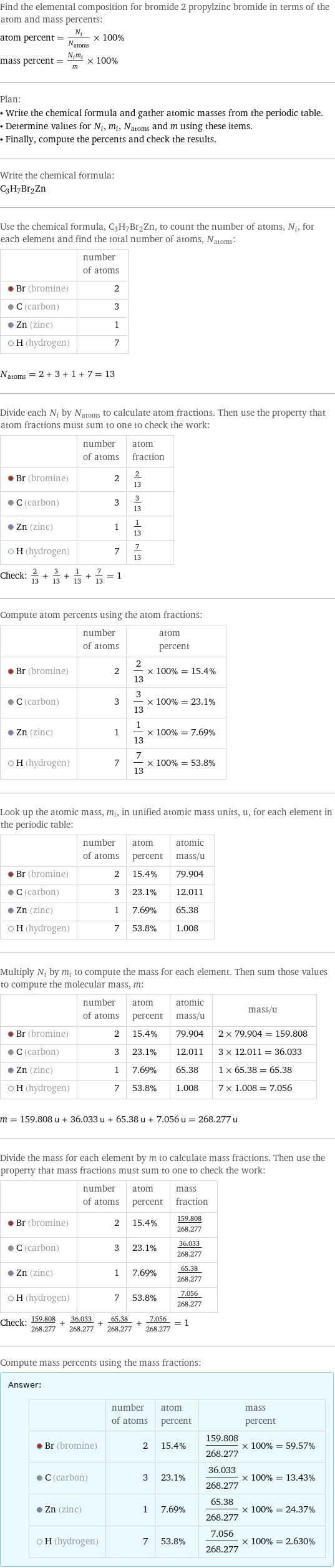
Find the elemental composition for bromide 2 propylzinc bromide in terms of the atom and mass percents: atom percent = N_i/N_atoms × 100% mass percent = (N_im_i)/m × 100% Plan: • Write the chemical formula and gather atomic masses from the periodic table. • Determine values for N_i, m_i, N_atoms and m using these items. • Finally, compute the percents and check the results. Write the chemical formula: C_3H_7Br_2Zn Use the chemical formula, C_3H_7Br_2Zn, to count the number of atoms, N_i, for each element and find the total number of atoms, N_atoms: | number of atoms Br (bromine) | 2 C (carbon) | 3 Zn (zinc) | 1 H (hydrogen) | 7 N_atoms = 2 + 3 + 1 + 7 = 13 Divide each N_i by N_atoms to calculate atom fractions. Then use the property that atom fractions must sum to one to check the work: | number of atoms | atom fraction Br (bromine) | 2 | 2/13 C (carbon) | 3 | 3/13 Zn (zinc) | 1 | 1/13 H (hydrogen) | 7 | 7/13 Check: 2/13 + 3/13 + 1/13 + 7/13 = 1 Compute atom percents using the atom fractions: | number of atoms | atom percent Br (bromine) | 2 | 2/13 × 100% = 15.4% C (carbon) | 3 | 3/13 × 100% = 23.1% Zn (zinc) | 1 | 1/13 × 100% = 7.69% H (hydrogen) | 7 | 7/13 × 100% = 53.8% Look up the atomic mass, m_i, in unified atomic mass units, u, for each element in the periodic table: | number of atoms | atom percent | atomic mass/u Br (bromine) | 2 | 15.4% | 79.904 C (carbon) | 3 | 23.1% | 12.011 Zn (zinc) | 1 | 7.69% | 65.38 H (hydrogen) | 7 | 53.8% | 1.008 Multiply N_i by m_i to compute the mass for each element. Then sum those values to compute the molecular mass, m: | number of atoms | atom percent | atomic mass/u | mass/u Br (bromine) | 2 | 15.4% | 79.904 | 2 × 79.904 = 159.808 C (carbon) | 3 | 23.1% | 12.011 | 3 × 12.011 = 36.033 Zn (zinc) | 1 | 7.69% | 65.38 | 1 × 65.38 = 65.38 H (hydrogen) | 7 | 53.8% | 1.008 | 7 × 1.008 = 7.056 m = 159.808 u + 36.033 u + 65.38 u + 7.056 u = 268.277 u Divide the mass for each element by m to calculate mass fractions. Then use the property that mass fractions must sum to one to check the work: | number of atoms | atom percent | mass fraction Br (bromine) | 2 | 15.4% | 159.808/268.277 C (carbon) | 3 | 23.1% | 36.033/268.277 Zn (zinc) | 1 | 7.69% | 65.38/268.277 H (hydrogen) | 7 | 53.8% | 7.056/268.277 Check: 159.808/268.277 + 36.033/268.277 + 65.38/268.277 + 7.056/268.277 = 1 Compute mass percents using the mass fractions: Answer: | | | number of atoms | atom percent | mass percent Br (bromine) | 2 | 15.4% | 159.808/268.277 × 100% = 59.57% C (carbon) | 3 | 23.1% | 36.033/268.277 × 100% = 13.43% Zn (zinc) | 1 | 7.69% | 65.38/268.277 × 100% = 24.37% H (hydrogen) | 7 | 53.8% | 7.056/268.277 × 100% = 2.630%
Topological indices
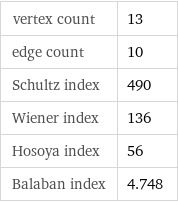
vertex count | 13 edge count | 10 Schultz index | 490 Wiener index | 136 Hosoya index | 56 Balaban index | 4.748