Input interpretation

Brønsted-Lowry bases | element types
Summary
bromine | carbon | chlorine | hydrogen | lithium | magnesium | nitrogen | sodium | oxygen
Periodic table location
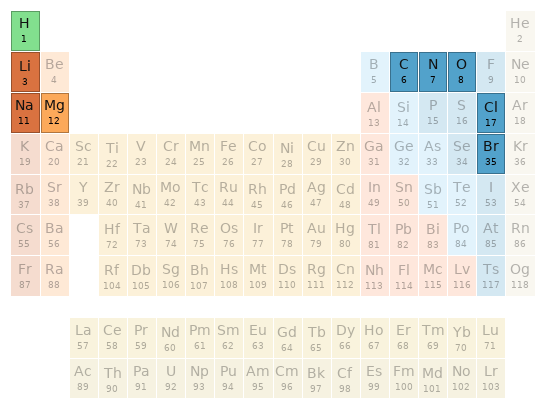
Periodic table location
Images

Images
Basic elemental properties
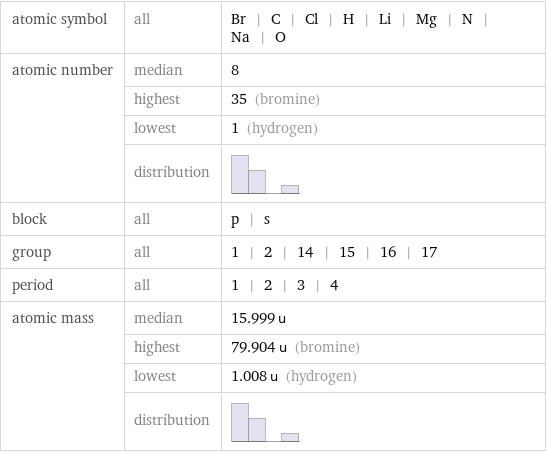
atomic symbol | all | Br | C | Cl | H | Li | Mg | N | Na | O atomic number | median | 8 | highest | 35 (bromine) | lowest | 1 (hydrogen) | distribution | block | all | p | s group | all | 1 | 2 | 14 | 15 | 16 | 17 period | all | 1 | 2 | 3 | 4 atomic mass | median | 15.999 u | highest | 79.904 u (bromine) | lowest | 1.008 u (hydrogen) | distribution |
Table
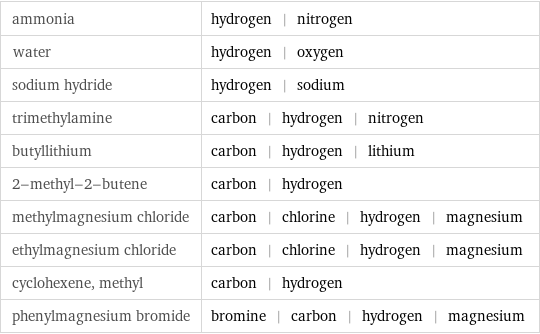
ammonia | hydrogen | nitrogen water | hydrogen | oxygen sodium hydride | hydrogen | sodium trimethylamine | carbon | hydrogen | nitrogen butyllithium | carbon | hydrogen | lithium 2-methyl-2-butene | carbon | hydrogen methylmagnesium chloride | carbon | chlorine | hydrogen | magnesium ethylmagnesium chloride | carbon | chlorine | hydrogen | magnesium cyclohexene, methyl | carbon | hydrogen phenylmagnesium bromide | bromine | carbon | hydrogen | magnesium
Thermodynamic properties
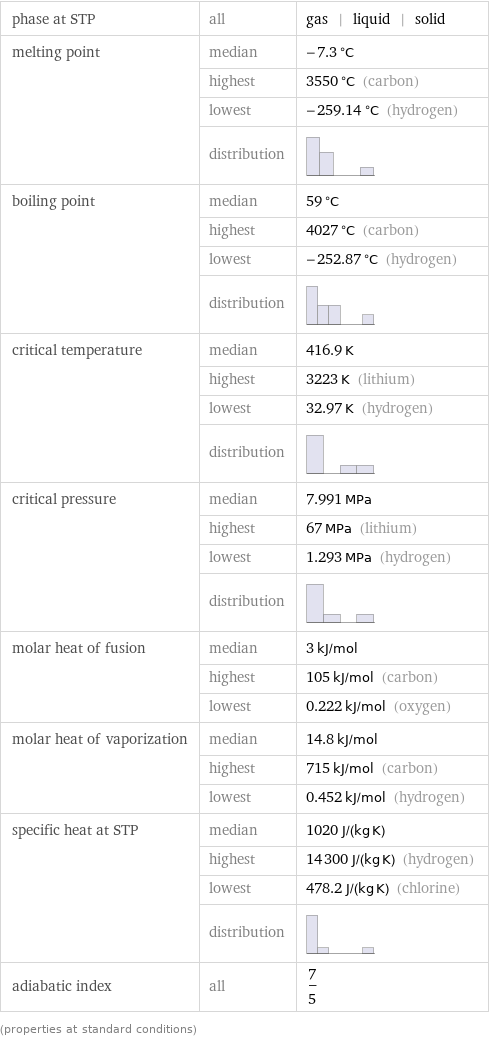
phase at STP | all | gas | liquid | solid melting point | median | -7.3 °C | highest | 3550 °C (carbon) | lowest | -259.14 °C (hydrogen) | distribution | boiling point | median | 59 °C | highest | 4027 °C (carbon) | lowest | -252.87 °C (hydrogen) | distribution | critical temperature | median | 416.9 K | highest | 3223 K (lithium) | lowest | 32.97 K (hydrogen) | distribution | critical pressure | median | 7.991 MPa | highest | 67 MPa (lithium) | lowest | 1.293 MPa (hydrogen) | distribution | molar heat of fusion | median | 3 kJ/mol | highest | 105 kJ/mol (carbon) | lowest | 0.222 kJ/mol (oxygen) molar heat of vaporization | median | 14.8 kJ/mol | highest | 715 kJ/mol (carbon) | lowest | 0.452 kJ/mol (hydrogen) specific heat at STP | median | 1020 J/(kg K) | highest | 14300 J/(kg K) (hydrogen) | lowest | 478.2 J/(kg K) (chlorine) | distribution | adiabatic index | all | 7/5 (properties at standard conditions)
Material properties
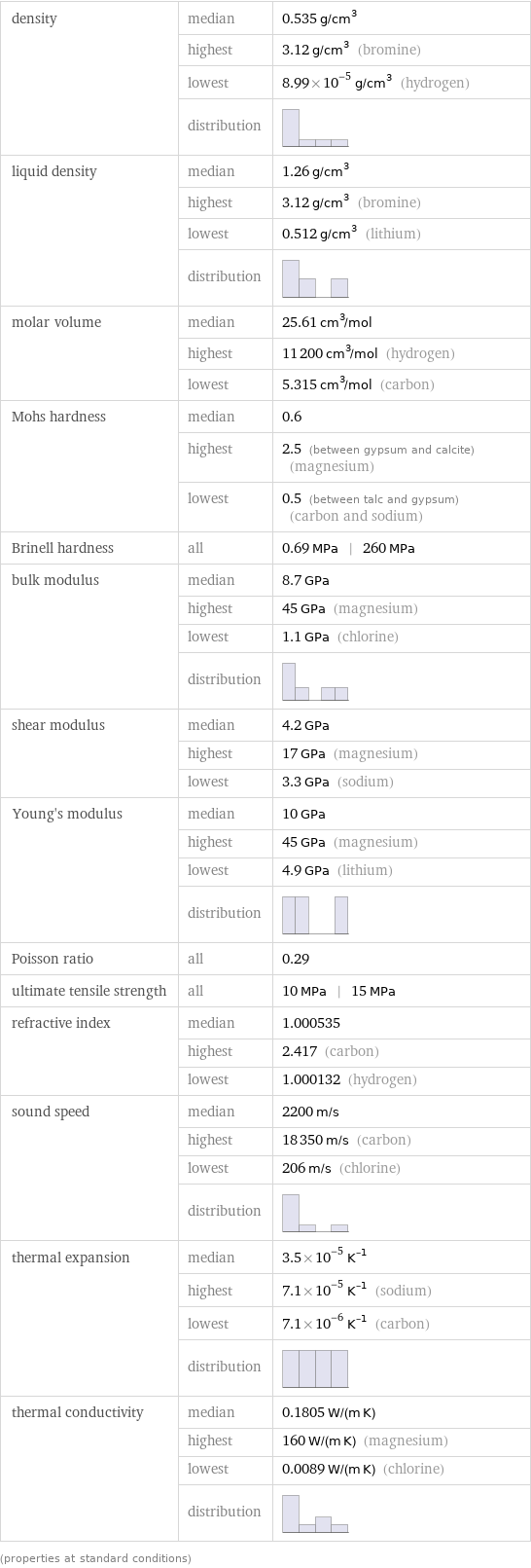
density | median | 0.535 g/cm^3 | highest | 3.12 g/cm^3 (bromine) | lowest | 8.99×10^-5 g/cm^3 (hydrogen) | distribution | liquid density | median | 1.26 g/cm^3 | highest | 3.12 g/cm^3 (bromine) | lowest | 0.512 g/cm^3 (lithium) | distribution | molar volume | median | 25.61 cm^3/mol | highest | 11200 cm^3/mol (hydrogen) | lowest | 5.315 cm^3/mol (carbon) Mohs hardness | median | 0.6 | highest | 2.5 (between gypsum and calcite) (magnesium) | lowest | 0.5 (between talc and gypsum) (carbon and sodium) Brinell hardness | all | 0.69 MPa | 260 MPa bulk modulus | median | 8.7 GPa | highest | 45 GPa (magnesium) | lowest | 1.1 GPa (chlorine) | distribution | shear modulus | median | 4.2 GPa | highest | 17 GPa (magnesium) | lowest | 3.3 GPa (sodium) Young's modulus | median | 10 GPa | highest | 45 GPa (magnesium) | lowest | 4.9 GPa (lithium) | distribution | Poisson ratio | all | 0.29 ultimate tensile strength | all | 10 MPa | 15 MPa refractive index | median | 1.000535 | highest | 2.417 (carbon) | lowest | 1.000132 (hydrogen) sound speed | median | 2200 m/s | highest | 18350 m/s (carbon) | lowest | 206 m/s (chlorine) | distribution | thermal expansion | median | 3.5×10^-5 K^(-1) | highest | 7.1×10^-5 K^(-1) (sodium) | lowest | 7.1×10^-6 K^(-1) (carbon) | distribution | thermal conductivity | median | 0.1805 W/(m K) | highest | 160 W/(m K) (magnesium) | lowest | 0.0089 W/(m K) (chlorine) | distribution | (properties at standard conditions)
Electromagnetic properties
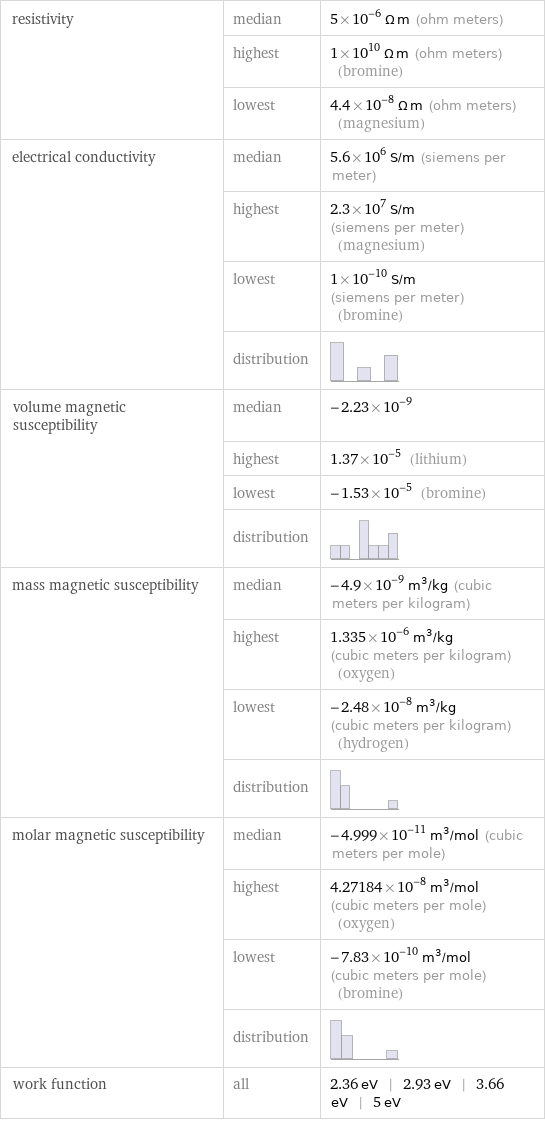
resistivity | median | 5×10^-6 Ω m (ohm meters) | highest | 1×10^10 Ω m (ohm meters) (bromine) | lowest | 4.4×10^-8 Ω m (ohm meters) (magnesium) electrical conductivity | median | 5.6×10^6 S/m (siemens per meter) | highest | 2.3×10^7 S/m (siemens per meter) (magnesium) | lowest | 1×10^-10 S/m (siemens per meter) (bromine) | distribution | volume magnetic susceptibility | median | -2.23×10^-9 | highest | 1.37×10^-5 (lithium) | lowest | -1.53×10^-5 (bromine) | distribution | mass magnetic susceptibility | median | -4.9×10^-9 m^3/kg (cubic meters per kilogram) | highest | 1.335×10^-6 m^3/kg (cubic meters per kilogram) (oxygen) | lowest | -2.48×10^-8 m^3/kg (cubic meters per kilogram) (hydrogen) | distribution | molar magnetic susceptibility | median | -4.999×10^-11 m^3/mol (cubic meters per mole) | highest | 4.27184×10^-8 m^3/mol (cubic meters per mole) (oxygen) | lowest | -7.83×10^-10 m^3/mol (cubic meters per mole) (bromine) | distribution | work function | all | 2.36 eV | 2.93 eV | 3.66 eV | 5 eV
Reactivity
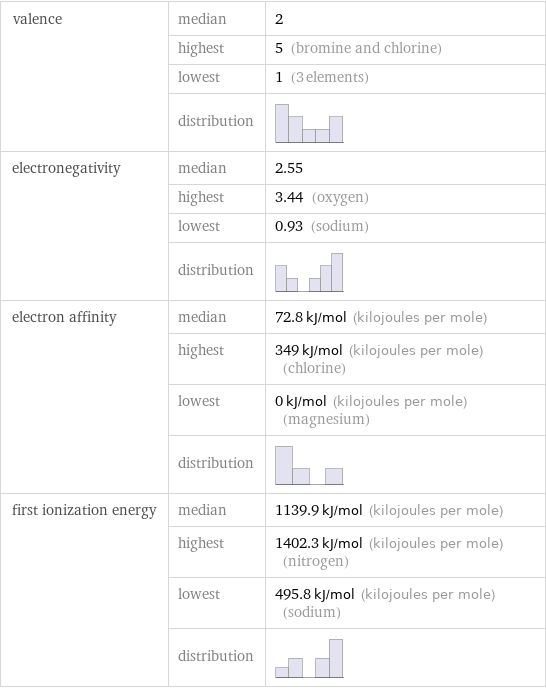
valence | median | 2 | highest | 5 (bromine and chlorine) | lowest | 1 (3 elements) | distribution | electronegativity | median | 2.55 | highest | 3.44 (oxygen) | lowest | 0.93 (sodium) | distribution | electron affinity | median | 72.8 kJ/mol (kilojoules per mole) | highest | 349 kJ/mol (kilojoules per mole) (chlorine) | lowest | 0 kJ/mol (kilojoules per mole) (magnesium) | distribution | first ionization energy | median | 1139.9 kJ/mol (kilojoules per mole) | highest | 1402.3 kJ/mol (kilojoules per mole) (nitrogen) | lowest | 495.8 kJ/mol (kilojoules per mole) (sodium) | distribution |
Atomic properties
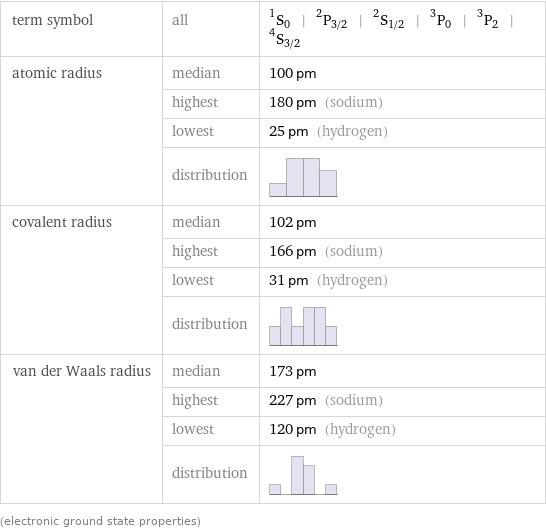
term symbol | all | ^1S_0 | ^2P_(3/2) | ^2S_(1/2) | ^3P_0 | ^3P_2 | ^4S_(3/2) atomic radius | median | 100 pm | highest | 180 pm (sodium) | lowest | 25 pm (hydrogen) | distribution | covalent radius | median | 102 pm | highest | 166 pm (sodium) | lowest | 31 pm (hydrogen) | distribution | van der Waals radius | median | 173 pm | highest | 227 pm (sodium) | lowest | 120 pm (hydrogen) | distribution | (electronic ground state properties)