Input interpretation
potassium nitrite | sodium amide
Chemical names and formulas
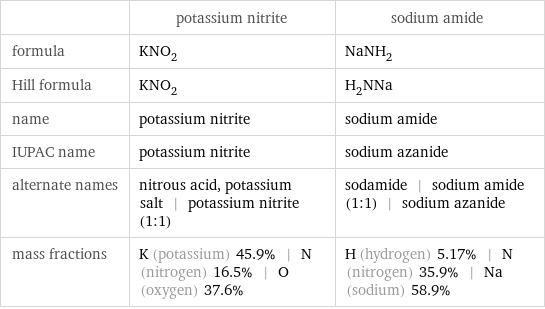
| potassium nitrite | sodium amide formula | KNO_2 | NaNH_2 Hill formula | KNO_2 | H_2NNa name | potassium nitrite | sodium amide IUPAC name | potassium nitrite | sodium azanide alternate names | nitrous acid, potassium salt | potassium nitrite (1:1) | sodamide | sodium amide (1:1) | sodium azanide mass fractions | K (potassium) 45.9% | N (nitrogen) 16.5% | O (oxygen) 37.6% | H (hydrogen) 5.17% | N (nitrogen) 35.9% | Na (sodium) 58.9%
Structure diagrams
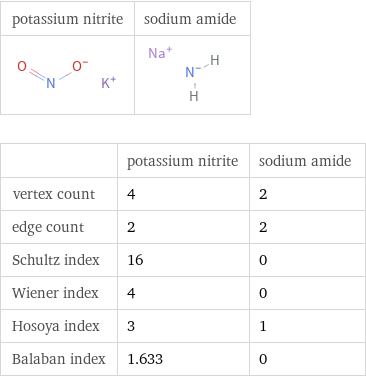
| potassium nitrite | sodium amide vertex count | 4 | 2 edge count | 2 | 2 Schultz index | 16 | 0 Wiener index | 4 | 0 Hosoya index | 3 | 1 Balaban index | 1.633 | 0
Basic properties
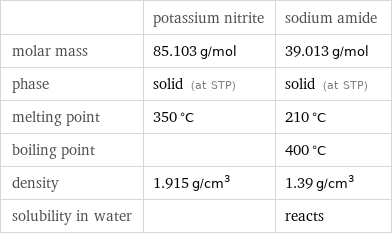
| potassium nitrite | sodium amide molar mass | 85.103 g/mol | 39.013 g/mol phase | solid (at STP) | solid (at STP) melting point | 350 °C | 210 °C boiling point | | 400 °C density | 1.915 g/cm^3 | 1.39 g/cm^3 solubility in water | | reacts
Units
