Input interpretation
4-fluoro-1-methyl-2-nitrobenzene
Chemical names and formulas
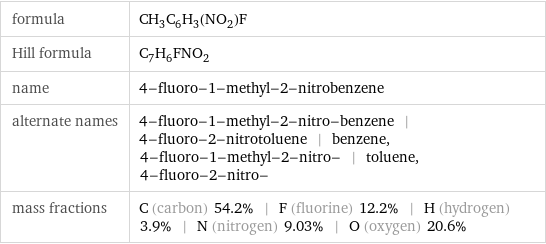
formula | CH_3C_6H_3(NO_2)F Hill formula | C_7H_6FNO_2 name | 4-fluoro-1-methyl-2-nitrobenzene alternate names | 4-fluoro-1-methyl-2-nitro-benzene | 4-fluoro-2-nitrotoluene | benzene, 4-fluoro-1-methyl-2-nitro- | toluene, 4-fluoro-2-nitro- mass fractions | C (carbon) 54.2% | F (fluorine) 12.2% | H (hydrogen) 3.9% | N (nitrogen) 9.03% | O (oxygen) 20.6%
Lewis structure
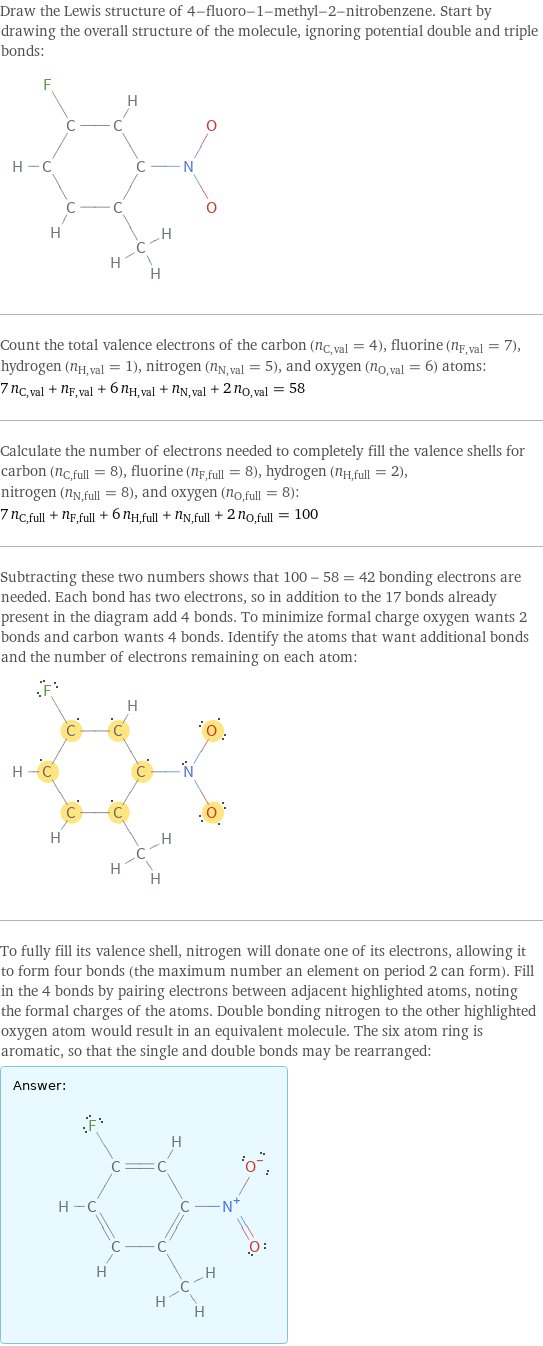
Draw the Lewis structure of 4-fluoro-1-methyl-2-nitrobenzene. Start by drawing the overall structure of the molecule, ignoring potential double and triple bonds: Count the total valence electrons of the carbon (n_C, val = 4), fluorine (n_F, val = 7), hydrogen (n_H, val = 1), nitrogen (n_N, val = 5), and oxygen (n_O, val = 6) atoms: 7 n_C, val + n_F, val + 6 n_H, val + n_N, val + 2 n_O, val = 58 Calculate the number of electrons needed to completely fill the valence shells for carbon (n_C, full = 8), fluorine (n_F, full = 8), hydrogen (n_H, full = 2), nitrogen (n_N, full = 8), and oxygen (n_O, full = 8): 7 n_C, full + n_F, full + 6 n_H, full + n_N, full + 2 n_O, full = 100 Subtracting these two numbers shows that 100 - 58 = 42 bonding electrons are needed. Each bond has two electrons, so in addition to the 17 bonds already present in the diagram add 4 bonds. To minimize formal charge oxygen wants 2 bonds and carbon wants 4 bonds. Identify the atoms that want additional bonds and the number of electrons remaining on each atom: To fully fill its valence shell, nitrogen will donate one of its electrons, allowing it to form four bonds (the maximum number an element on period 2 can form). Fill in the 4 bonds by pairing electrons between adjacent highlighted atoms, noting the formal charges of the atoms. Double bonding nitrogen to the other highlighted oxygen atom would result in an equivalent molecule. The six atom ring is aromatic, so that the single and double bonds may be rearranged: Answer: | |
3D structure
3D structure
Basic properties
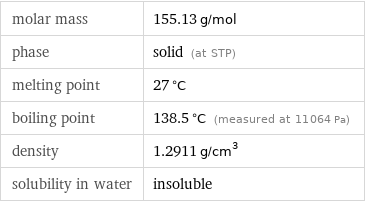
molar mass | 155.13 g/mol phase | solid (at STP) melting point | 27 °C boiling point | 138.5 °C (measured at 11064 Pa) density | 1.2911 g/cm^3 solubility in water | insoluble
Units

Solid properties (at STP)
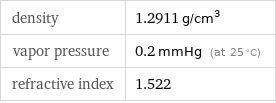
density | 1.2911 g/cm^3 vapor pressure | 0.2 mmHg (at 25 °C) refractive index | 1.522
Units

Thermodynamic properties

molar heat of vaporization | 43.2 kJ/mol specific heat of vaporization | 0.278 kJ/g (at STP)
Chemical identifiers
[O-] InChI identifier | InChI=1/C7H6FNO2/c1-5-2-3-6(8)4-7(5)9(10)11/h2-4H, 1H3 MDL number | MFCD00007200](../image_source/c679ed302b3c3c01b4fee1c86f873c1c.png)
CAS number | 446-10-6 PubChem CID number | 67965 PubChem SID number | 24894755 SMILES identifier | CC1=C(C=C(C=C1)F)[N+](=O)[O-] InChI identifier | InChI=1/C7H6FNO2/c1-5-2-3-6(8)4-7(5)9(10)11/h2-4H, 1H3 MDL number | MFCD00007200
NFPA label

NFPA label

NFPA health rating | 2 NFPA fire rating | 1 NFPA reactivity rating | 0
Safety properties

flash point | 98.89 °C

DOT hazard class | 6.1 DOT numbers | 2810