Input interpretation
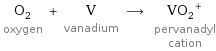
O_2 oxygen + V vanadium ⟶ (VO_2)^+ pervanadyl cation
Chemical names and formulas

| oxygen | vanadium | pervanadyl cation formula | O_2 | V | (VO_2)^+ name | oxygen | vanadium | pervanadyl cation IUPAC name | molecular oxygen | vanadium |
Substance properties
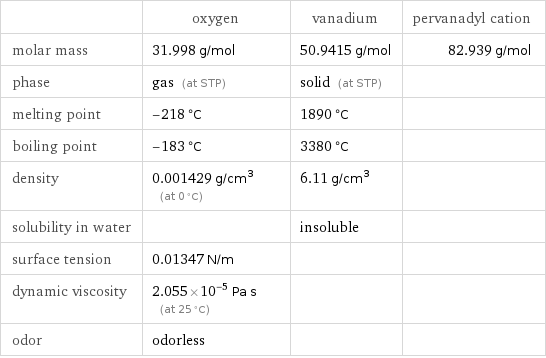
| oxygen | vanadium | pervanadyl cation molar mass | 31.998 g/mol | 50.9415 g/mol | 82.939 g/mol phase | gas (at STP) | solid (at STP) | melting point | -218 °C | 1890 °C | boiling point | -183 °C | 3380 °C | density | 0.001429 g/cm^3 (at 0 °C) | 6.11 g/cm^3 | solubility in water | | insoluble | surface tension | 0.01347 N/m | | dynamic viscosity | 2.055×10^-5 Pa s (at 25 °C) | | odor | odorless | |
Units
