Input interpretation
halogens | phase at STP
Summary
gas | liquid | solid (based on 5 values; 1 unavailable)
Entity with missing value
tennessine
Table
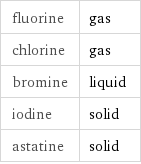
fluorine | gas chlorine | gas bromine | liquid iodine | solid astatine | solid
Thermodynamic properties
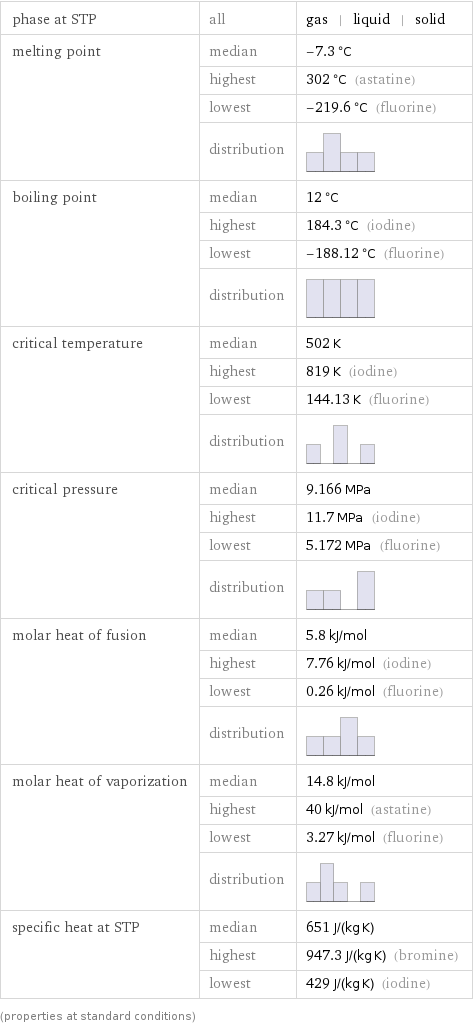
phase at STP | all | gas | liquid | solid melting point | median | -7.3 °C | highest | 302 °C (astatine) | lowest | -219.6 °C (fluorine) | distribution | boiling point | median | 12 °C | highest | 184.3 °C (iodine) | lowest | -188.12 °C (fluorine) | distribution | critical temperature | median | 502 K | highest | 819 K (iodine) | lowest | 144.13 K (fluorine) | distribution | critical pressure | median | 9.166 MPa | highest | 11.7 MPa (iodine) | lowest | 5.172 MPa (fluorine) | distribution | molar heat of fusion | median | 5.8 kJ/mol | highest | 7.76 kJ/mol (iodine) | lowest | 0.26 kJ/mol (fluorine) | distribution | molar heat of vaporization | median | 14.8 kJ/mol | highest | 40 kJ/mol (astatine) | lowest | 3.27 kJ/mol (fluorine) | distribution | specific heat at STP | median | 651 J/(kg K) | highest | 947.3 J/(kg K) (bromine) | lowest | 429 J/(kg K) (iodine) (properties at standard conditions)