Input interpretation

naturally occurring elements | van der Waals radius
Summary
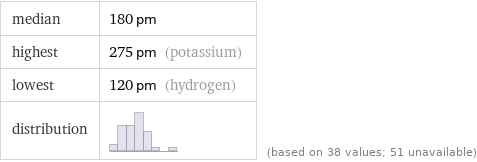
median | 180 pm highest | 275 pm (potassium) lowest | 120 pm (hydrogen) distribution | | (based on 38 values; 51 unavailable)
Entities with missing values
actinium | aluminum | antimony | barium | beryllium | bismuth | boron | calcium | cerium | cesium | ... (total: 51)
Plot
![ListPlot[{{{18, 188.}, {33, 185.}, {35, 185.}, {48, 158.}, {6, 170.}, {17, 175.}, {29, 140.}, {9, 147.}, {31, 187.}, {79, 166.}, {2, 140.}, {1, 120.}, {49, 193.}, {53, 198.}, {36, 202.}, {82, 202.}, {3, 182.}, {12, 173.}, {80, 155.}, {10, 154.}, {28, 163.}, {7, 155.}, {8, 152.}, {46, 163.}, {15, 180.}, {78, 175.}, {19, 275.}, {34, 190.}, {14, 210.}, {47, 172.}, {11, 227.}, {16, 180.}, {52, 206.}, {81, 196.}, {50, 217.}, {92, 186.}, {54, 216.}, {30, 139.}}, {}}, Filling->Bottom, PlotRange->Full, Axes->False, Frame->{True, True, None, None}, PlotStyle->{, }, FillingStyle->{1->{Directive[, AbsoluteThickness[1]]}, 2->{}}, PlotRangePadding->{{1, 1}, None}, PlotRangeClipping->True, FrameLabel->atomic number | van der Waals radius (in picometers), ImageSize->Full]](../image_source/85d93a6f9140883485c12b9d30737b45.png)
ListPlot[{{{18, 188.}, {33, 185.}, {35, 185.}, {48, 158.}, {6, 170.}, {17, 175.}, {29, 140.}, {9, 147.}, {31, 187.}, {79, 166.}, {2, 140.}, {1, 120.}, {49, 193.}, {53, 198.}, {36, 202.}, {82, 202.}, {3, 182.}, {12, 173.}, {80, 155.}, {10, 154.}, {28, 163.}, {7, 155.}, {8, 152.}, {46, 163.}, {15, 180.}, {78, 175.}, {19, 275.}, {34, 190.}, {14, 210.}, {47, 172.}, {11, 227.}, {16, 180.}, {52, 206.}, {81, 196.}, {50, 217.}, {92, 186.}, {54, 216.}, {30, 139.}}, {}}, Filling->Bottom, PlotRange->Full, Axes->False, Frame->{True, True, None, None}, PlotStyle->{, }, FillingStyle->{1->{Directive[, AbsoluteThickness[1]]}, 2->{}}, PlotRangePadding->{{1, 1}, None}, PlotRangeClipping->True, FrameLabel->atomic number | van der Waals radius (in picometers), ImageSize->Full]
Periodic table location
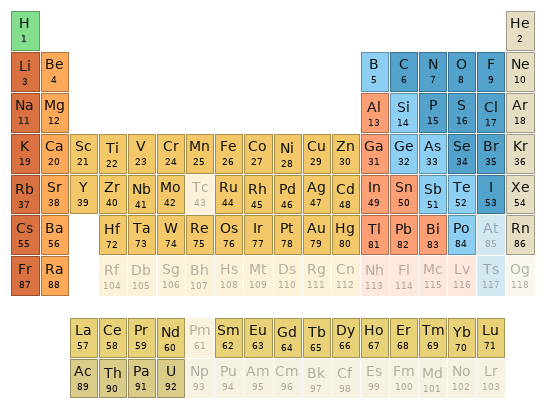
Periodic table location
Atomic radii properties
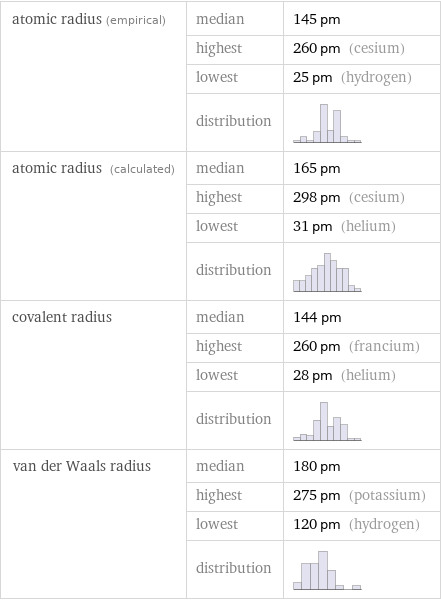
atomic radius (empirical) | median | 145 pm | highest | 260 pm (cesium) | lowest | 25 pm (hydrogen) | distribution | atomic radius (calculated) | median | 165 pm | highest | 298 pm (cesium) | lowest | 31 pm (helium) | distribution | covalent radius | median | 144 pm | highest | 260 pm (francium) | lowest | 28 pm (helium) | distribution | van der Waals radius | median | 180 pm | highest | 275 pm (potassium) | lowest | 120 pm (hydrogen) | distribution |
Units

Distribution plots
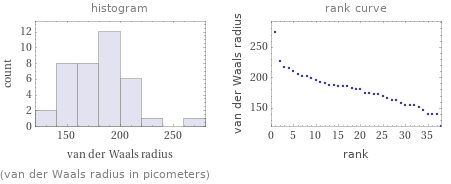
(van der Waals radius in picometers)
Van der Waals radius rankings
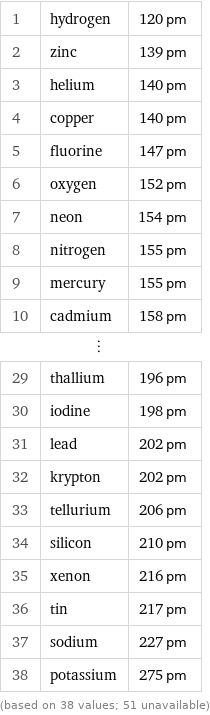
1 | hydrogen | 120 pm 2 | zinc | 139 pm 3 | helium | 140 pm 4 | copper | 140 pm 5 | fluorine | 147 pm 6 | oxygen | 152 pm 7 | neon | 154 pm 8 | nitrogen | 155 pm 9 | mercury | 155 pm 10 | cadmium | 158 pm ⋮ | | 29 | thallium | 196 pm 30 | iodine | 198 pm 31 | lead | 202 pm 32 | krypton | 202 pm 33 | tellurium | 206 pm 34 | silicon | 210 pm 35 | xenon | 216 pm 36 | tin | 217 pm 37 | sodium | 227 pm 38 | potassium | 275 pm (based on 38 values; 51 unavailable)
Unit conversions for median van der Waals radius 180 pm
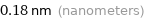
0.18 nm (nanometers)
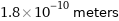
1.8×10^-10 meters
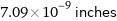
7.09×10^-9 inches
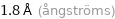
1.8 Å (ångströms)
Comparisons for median van der Waals radius 180 pm
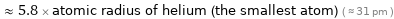
≈ 5.8 × atomic radius of helium (the smallest atom) ( ≈ 31 pm )
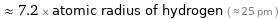
≈ 7.2 × atomic radius of hydrogen ( ≈ 25 pm )