Input interpretation
solid compounds
Members

acetamide | acetoxime | aluminum | beryllium | beryllium oxide | lime | cobalt | copper | iron | lithium | lithium fluoride | magnesium oxide | potassium fluoride | quartz | scandium | sodium amide | sodium fluoride | vanadium | vanadium(II) oxide | zinc (total: 20)
Basic properties
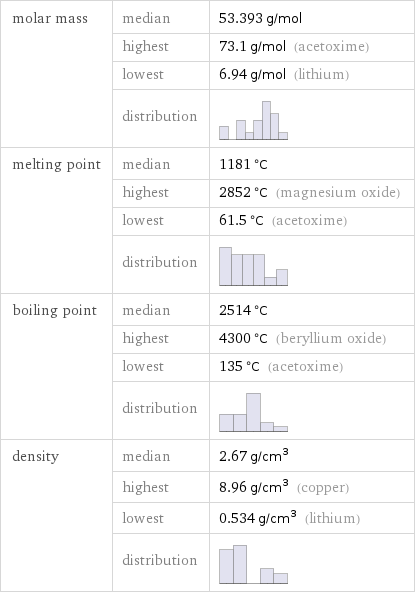
molar mass | median | 53.393 g/mol | highest | 73.1 g/mol (acetoxime) | lowest | 6.94 g/mol (lithium) | distribution | melting point | median | 1181 °C | highest | 2852 °C (magnesium oxide) | lowest | 61.5 °C (acetoxime) | distribution | boiling point | median | 2514 °C | highest | 4300 °C (beryllium oxide) | lowest | 135 °C (acetoxime) | distribution | density | median | 2.67 g/cm^3 | highest | 8.96 g/cm^3 (copper) | lowest | 0.534 g/cm^3 (lithium) | distribution |
Units
