Input interpretation

paramagnetic elements | atomic mass
Summary
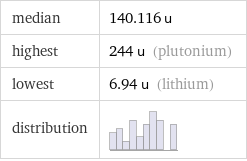
median | 140.116 u highest | 244 u (plutonium) lowest | 6.94 u (lithium) distribution |
Units

Distribution plots
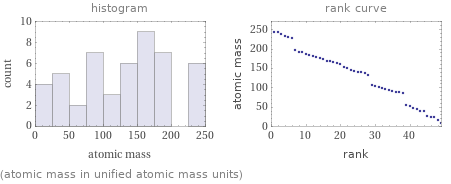
(atomic mass in unified atomic mass units)
Atomic mass rankings
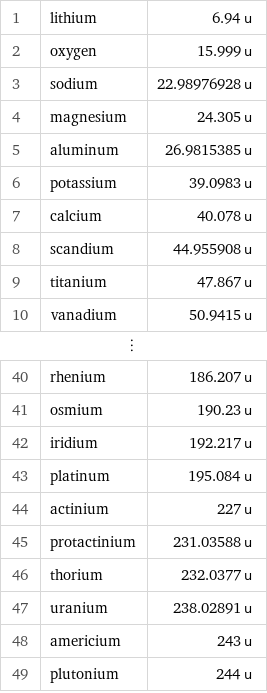
1 | lithium | 6.94 u 2 | oxygen | 15.999 u 3 | sodium | 22.98976928 u 4 | magnesium | 24.305 u 5 | aluminum | 26.9815385 u 6 | potassium | 39.0983 u 7 | calcium | 40.078 u 8 | scandium | 44.955908 u 9 | titanium | 47.867 u 10 | vanadium | 50.9415 u ⋮ | | 40 | rhenium | 186.207 u 41 | osmium | 190.23 u 42 | iridium | 192.217 u 43 | platinum | 195.084 u 44 | actinium | 227 u 45 | protactinium | 231.03588 u 46 | thorium | 232.0377 u 47 | uranium | 238.02891 u 48 | americium | 243 u 49 | plutonium | 244 u
Unit conversions for median atomic mass 140.116 u
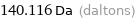
140.116 Da (daltons)
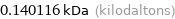
0.140116 kDa (kilodaltons)
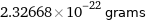
2.32668×10^-22 grams
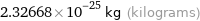
2.32668×10^-25 kg (kilograms)

140.122 chemical atomic mass units (unit officially deprecated)

140.161 physical atomic mass units (unit officially deprecated)
Corresponding quantities
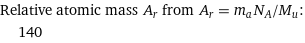
Relative atomic mass A_r from A_r = m_aN_A/M_u: | 140
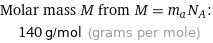
Molar mass M from M = m_aN_A: | 140 g/mol (grams per mole)