Input interpretation

period 4 elements | first molar ionization energy
Summary
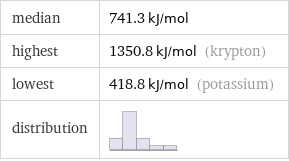
median | 741.3 kJ/mol highest | 1350.8 kJ/mol (krypton) lowest | 418.8 kJ/mol (potassium) distribution |
Units

Distribution plots
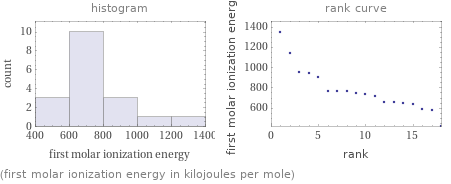
(first molar ionization energy in kilojoules per mole)
First molar ionization energy rankings
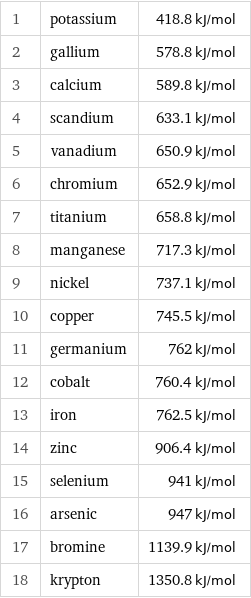
1 | potassium | 418.8 kJ/mol 2 | gallium | 578.8 kJ/mol 3 | calcium | 589.8 kJ/mol 4 | scandium | 633.1 kJ/mol 5 | vanadium | 650.9 kJ/mol 6 | chromium | 652.9 kJ/mol 7 | titanium | 658.8 kJ/mol 8 | manganese | 717.3 kJ/mol 9 | nickel | 737.1 kJ/mol 10 | copper | 745.5 kJ/mol 11 | germanium | 762 kJ/mol 12 | cobalt | 760.4 kJ/mol 13 | iron | 762.5 kJ/mol 14 | zinc | 906.4 kJ/mol 15 | selenium | 941 kJ/mol 16 | arsenic | 947 kJ/mol 17 | bromine | 1139.9 kJ/mol 18 | krypton | 1350.8 kJ/mol
Unit conversions for median first molar ionization energy 741.3 kJ/mol
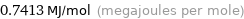
0.7413 MJ/mol (megajoules per mole)
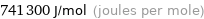
741300 J/mol (joules per mole)
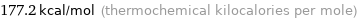
177.2 kcal/mol (thermochemical kilocalories per mole)

7.683 eV (molar electronvolts)
Comparisons for median first molar ionization energy 741.3 kJ/mol

≈ 0.31 × molar energy required to remove the first electron from helium ( 2372 kJ/mol )
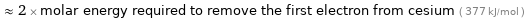
≈ 2 × molar energy required to remove the first electron from cesium ( 377 kJ/mol )