Input interpretation

actinium (chemical element) | cerium (chemical element) | terbium (chemical element)
Periodic table location
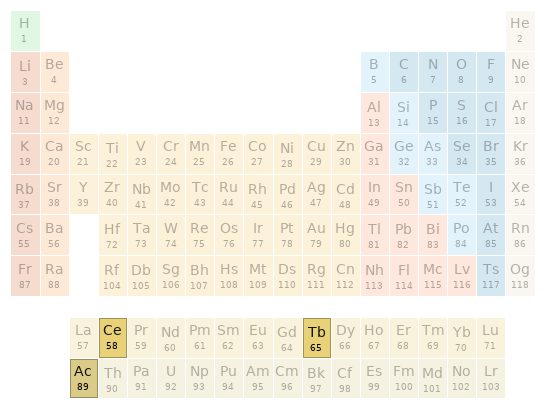
Periodic table location
Images
Images
Basic elemental properties
![| actinium | cerium | terbium atomic symbol | Ac | Ce | Tb atomic number | 89 | 58 | 65 short electronic configuration | [Rn]7s^26d^1 | [Xe]6s^24f^15d^1 | [Xe]6s^24f^9 Aufbau diagram | 6d 7s | 5d 4f 6s | 4f 6s block | f | f | f period | 7 | 6 | 6 atomic mass | 227 u | 140.116 u | 158.92535 u half-life | 21.787 yr | (stable) | (stable)](../image_source/e59469454e95290539b2803112ec81d7.png)
| actinium | cerium | terbium atomic symbol | Ac | Ce | Tb atomic number | 89 | 58 | 65 short electronic configuration | [Rn]7s^26d^1 | [Xe]6s^24f^15d^1 | [Xe]6s^24f^9 Aufbau diagram | 6d 7s | 5d 4f 6s | 4f 6s block | f | f | f period | 7 | 6 | 6 atomic mass | 227 u | 140.116 u | 158.92535 u half-life | 21.787 yr | (stable) | (stable)
Thermodynamic properties
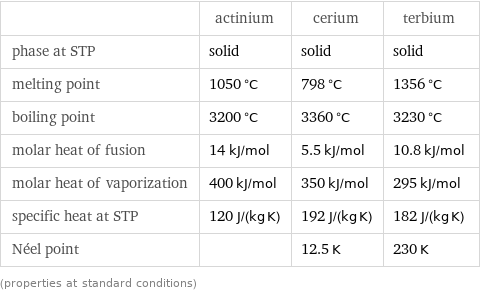
| actinium | cerium | terbium phase at STP | solid | solid | solid melting point | 1050 °C | 798 °C | 1356 °C boiling point | 3200 °C | 3360 °C | 3230 °C molar heat of fusion | 14 kJ/mol | 5.5 kJ/mol | 10.8 kJ/mol molar heat of vaporization | 400 kJ/mol | 350 kJ/mol | 295 kJ/mol specific heat at STP | 120 J/(kg K) | 192 J/(kg K) | 182 J/(kg K) Néel point | | 12.5 K | 230 K (properties at standard conditions)
Material properties
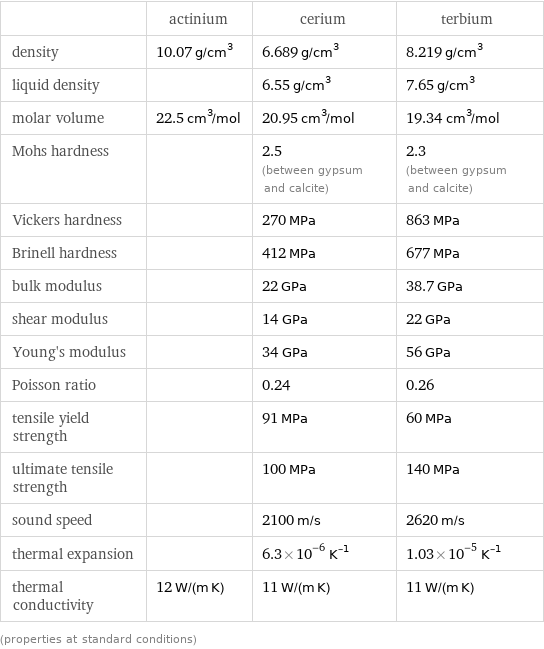
| actinium | cerium | terbium density | 10.07 g/cm^3 | 6.689 g/cm^3 | 8.219 g/cm^3 liquid density | | 6.55 g/cm^3 | 7.65 g/cm^3 molar volume | 22.5 cm^3/mol | 20.95 cm^3/mol | 19.34 cm^3/mol Mohs hardness | | 2.5 (between gypsum and calcite) | 2.3 (between gypsum and calcite) Vickers hardness | | 270 MPa | 863 MPa Brinell hardness | | 412 MPa | 677 MPa bulk modulus | | 22 GPa | 38.7 GPa shear modulus | | 14 GPa | 22 GPa Young's modulus | | 34 GPa | 56 GPa Poisson ratio | | 0.24 | 0.26 tensile yield strength | | 91 MPa | 60 MPa ultimate tensile strength | | 100 MPa | 140 MPa sound speed | | 2100 m/s | 2620 m/s thermal expansion | | 6.3×10^-6 K^(-1) | 1.03×10^-5 K^(-1) thermal conductivity | 12 W/(m K) | 11 W/(m K) | 11 W/(m K) (properties at standard conditions)
Electromagnetic properties
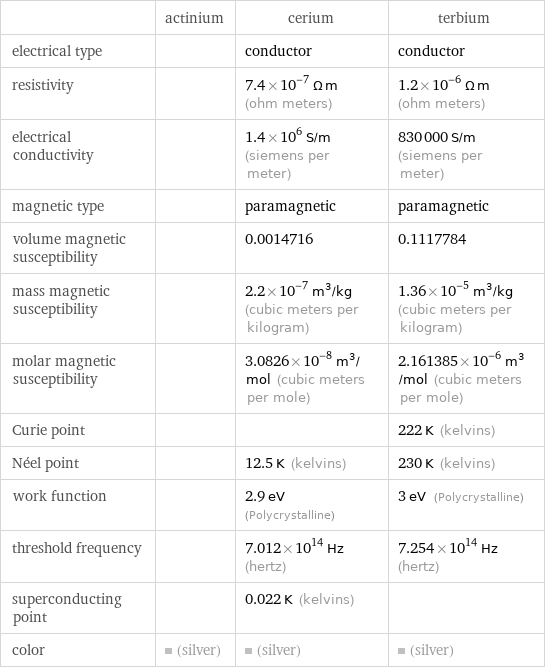
| actinium | cerium | terbium electrical type | | conductor | conductor resistivity | | 7.4×10^-7 Ω m (ohm meters) | 1.2×10^-6 Ω m (ohm meters) electrical conductivity | | 1.4×10^6 S/m (siemens per meter) | 830000 S/m (siemens per meter) magnetic type | | paramagnetic | paramagnetic volume magnetic susceptibility | | 0.0014716 | 0.1117784 mass magnetic susceptibility | | 2.2×10^-7 m^3/kg (cubic meters per kilogram) | 1.36×10^-5 m^3/kg (cubic meters per kilogram) molar magnetic susceptibility | | 3.0826×10^-8 m^3/mol (cubic meters per mole) | 2.161385×10^-6 m^3/mol (cubic meters per mole) Curie point | | | 222 K (kelvins) Néel point | | 12.5 K (kelvins) | 230 K (kelvins) work function | | 2.9 eV (Polycrystalline) | 3 eV (Polycrystalline) threshold frequency | | 7.012×10^14 Hz (hertz) | 7.254×10^14 Hz (hertz) superconducting point | | 0.022 K (kelvins) | color | (silver) | (silver) | (silver)
Reactivity
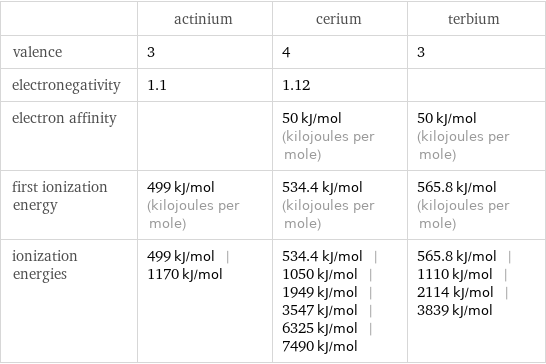
| actinium | cerium | terbium valence | 3 | 4 | 3 electronegativity | 1.1 | 1.12 | electron affinity | | 50 kJ/mol (kilojoules per mole) | 50 kJ/mol (kilojoules per mole) first ionization energy | 499 kJ/mol (kilojoules per mole) | 534.4 kJ/mol (kilojoules per mole) | 565.8 kJ/mol (kilojoules per mole) ionization energies | 499 kJ/mol | 1170 kJ/mol | 534.4 kJ/mol | 1050 kJ/mol | 1949 kJ/mol | 3547 kJ/mol | 6325 kJ/mol | 7490 kJ/mol | 565.8 kJ/mol | 1110 kJ/mol | 2114 kJ/mol | 3839 kJ/mol
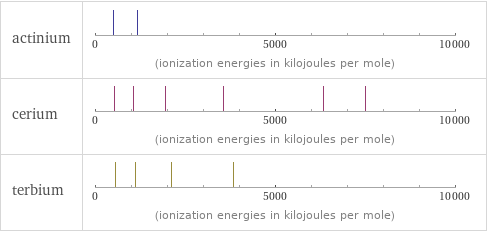
Reactivity
Atomic properties
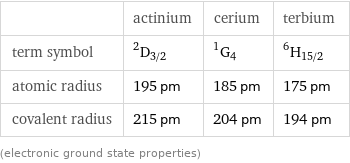
| actinium | cerium | terbium term symbol | ^2D_(3/2) | ^1G_4 | ^6H_(15/2) atomic radius | 195 pm | 185 pm | 175 pm covalent radius | 215 pm | 204 pm | 194 pm (electronic ground state properties)
Abundances
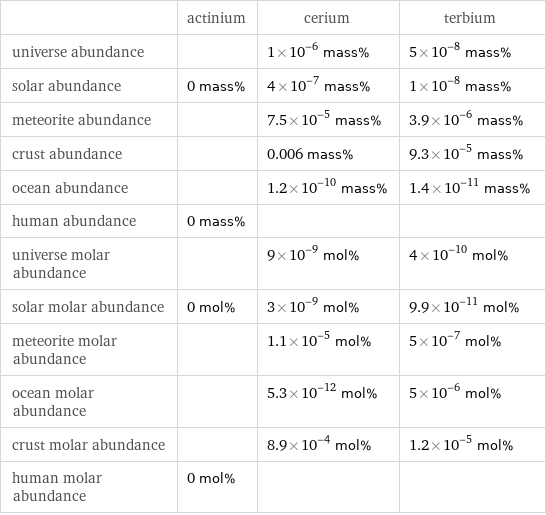
| actinium | cerium | terbium universe abundance | | 1×10^-6 mass% | 5×10^-8 mass% solar abundance | 0 mass% | 4×10^-7 mass% | 1×10^-8 mass% meteorite abundance | | 7.5×10^-5 mass% | 3.9×10^-6 mass% crust abundance | | 0.006 mass% | 9.3×10^-5 mass% ocean abundance | | 1.2×10^-10 mass% | 1.4×10^-11 mass% human abundance | 0 mass% | | universe molar abundance | | 9×10^-9 mol% | 4×10^-10 mol% solar molar abundance | 0 mol% | 3×10^-9 mol% | 9.9×10^-11 mol% meteorite molar abundance | | 1.1×10^-5 mol% | 5×10^-7 mol% ocean molar abundance | | 5.3×10^-12 mol% | 5×10^-6 mol% crust molar abundance | | 8.9×10^-4 mol% | 1.2×10^-5 mol% human molar abundance | 0 mol% | |
Nuclear properties
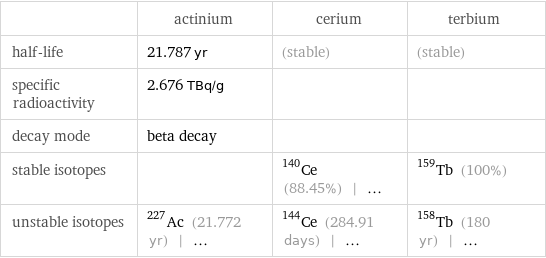
| actinium | cerium | terbium half-life | 21.787 yr | (stable) | (stable) specific radioactivity | 2.676 TBq/g | | decay mode | beta decay | | stable isotopes | | Ce-140 (88.45%) | ... | Tb-159 (100%) unstable isotopes | Ac-227 (21.772 yr) | ... | Ce-144 (284.91 days) | ... | Tb-158 (180 yr) | ...
Units