Input interpretation
potassium nitrate
Chemical names and formulas
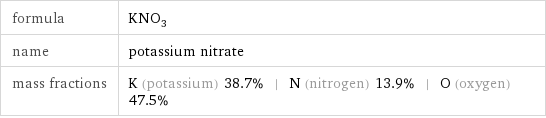
formula | KNO_3 name | potassium nitrate mass fractions | K (potassium) 38.7% | N (nitrogen) 13.9% | O (oxygen) 47.5%
Structure diagram
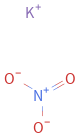
Structure diagram
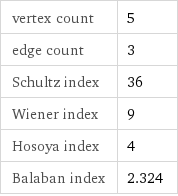
vertex count | 5 edge count | 3 Schultz index | 36 Wiener index | 9 Hosoya index | 4 Balaban index | 2.324
Basic properties
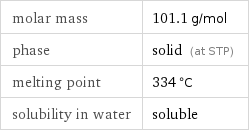
molar mass | 101.1 g/mol phase | solid (at STP) melting point | 334 °C solubility in water | soluble
Units

Thermodynamic properties
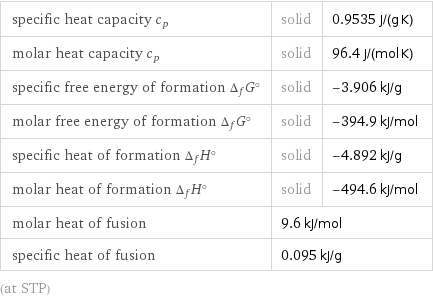
specific heat capacity c_p | solid | 0.9535 J/(g K) molar heat capacity c_p | solid | 96.4 J/(mol K) specific free energy of formation Δ_fG° | solid | -3.906 kJ/g molar free energy of formation Δ_fG° | solid | -394.9 kJ/mol specific heat of formation Δ_fH° | solid | -4.892 kJ/g molar heat of formation Δ_fH° | solid | -494.6 kJ/mol molar heat of fusion | 9.6 kJ/mol | specific heat of fusion | 0.095 kJ/g | (at STP)
Chemical identifiers
([O-])[O-].[K+] InChI identifier | InChI=1/K.NO3/c;2-1(3)4/q+1;-1 RTECS number | TT3700000 MDL number | MFCD00011409](../image_source/4b90ace71b4e8ac3fceb2b948f093283.png)
CAS number | 7757-79-1 PubChem CID number | 24434 PubChem SID number | 24852201 SMILES identifier | [N+](=O)([O-])[O-].[K+] InChI identifier | InChI=1/K.NO3/c;2-1(3)4/q+1;-1 RTECS number | TT3700000 MDL number | MFCD00011409
Toxicity properties
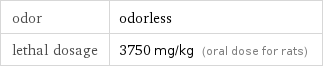
odor | odorless lethal dosage | 3750 mg/kg (oral dose for rats)
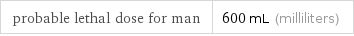
probable lethal dose for man | 600 mL (milliliters)
Ion equivalents

K^+ (potassium cation) | 1 (NO_3)^- (nitrate anion) | 1