Input interpretation
pyrite
Chemical names and formulas

formula | FeS_2 name | pyrite IUPAC name | bis(sulfanylidene)iron alternate names | iron disulfide | iron pyrite | iron sulfide mass fractions | Fe (iron) 46.6% | S (sulfur) 53.4%
Structure diagram
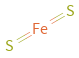
Structure diagram
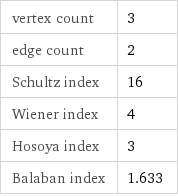
vertex count | 3 edge count | 2 Schultz index | 16 Wiener index | 4 Hosoya index | 3 Balaban index | 1.633
Basic properties

molar mass | 120 g/mol density | 4.89 g/cm^3
Units

Thermodynamic properties
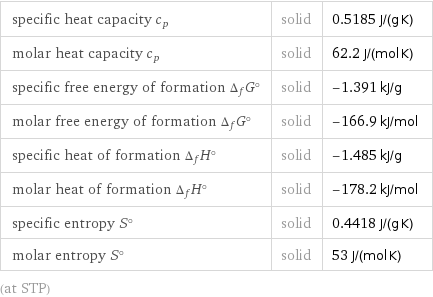
specific heat capacity c_p | solid | 0.5185 J/(g K) molar heat capacity c_p | solid | 62.2 J/(mol K) specific free energy of formation Δ_fG° | solid | -1.391 kJ/g molar free energy of formation Δ_fG° | solid | -166.9 kJ/mol specific heat of formation Δ_fH° | solid | -1.485 kJ/g molar heat of formation Δ_fH° | solid | -178.2 kJ/mol specific entropy S° | solid | 0.4418 J/(g K) molar entropy S° | solid | 53 J/(mol K) (at STP)
Chemical identifiers
![CAS number | 1309-36-0 PubChem CID number | 14788 SMILES identifier | S=[Fe]=S EU number | 215-167-7 Gmelin number | 228601](../image_source/92397497ae3c6ce71a5cd414c4a45854.png)
CAS number | 1309-36-0 PubChem CID number | 14788 SMILES identifier | S=[Fe]=S EU number | 215-167-7 Gmelin number | 228601
Toxicity properties

odor | odorless