Input interpretation
phytonadione
Chemical names and formulas
![formula | C_31H_46O_2 name | phytonadione IUPAC name | 3-methyl-2-[(E)-3, 7, 11, 15-tetramethylhexadec-2-enyl]naphthalene-1, 4-dione alternate names | 2-methyl-3-phytyl-1, 4-naphthoquinone mass fractions | C (carbon) 82.6% | H (hydrogen) 10.3% | O (oxygen) 7.1%](../image_source/3a858015e59401d29cdeeaf2df0e6fbc.png)
formula | C_31H_46O_2 name | phytonadione IUPAC name | 3-methyl-2-[(E)-3, 7, 11, 15-tetramethylhexadec-2-enyl]naphthalene-1, 4-dione alternate names | 2-methyl-3-phytyl-1, 4-naphthoquinone mass fractions | C (carbon) 82.6% | H (hydrogen) 10.3% | O (oxygen) 7.1%
Lewis structure
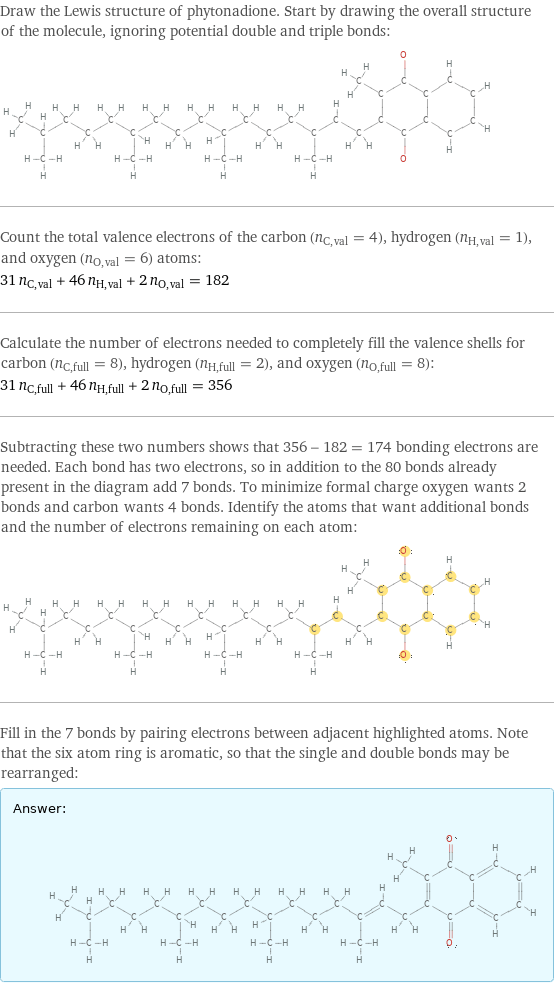
Draw the Lewis structure of phytonadione. Start by drawing the overall structure of the molecule, ignoring potential double and triple bonds: Count the total valence electrons of the carbon (n_C, val = 4), hydrogen (n_H, val = 1), and oxygen (n_O, val = 6) atoms: 31 n_C, val + 46 n_H, val + 2 n_O, val = 182 Calculate the number of electrons needed to completely fill the valence shells for carbon (n_C, full = 8), hydrogen (n_H, full = 2), and oxygen (n_O, full = 8): 31 n_C, full + 46 n_H, full + 2 n_O, full = 356 Subtracting these two numbers shows that 356 - 182 = 174 bonding electrons are needed. Each bond has two electrons, so in addition to the 80 bonds already present in the diagram add 7 bonds. To minimize formal charge oxygen wants 2 bonds and carbon wants 4 bonds. Identify the atoms that want additional bonds and the number of electrons remaining on each atom: Fill in the 7 bonds by pairing electrons between adjacent highlighted atoms. Note that the six atom ring is aromatic, so that the single and double bonds may be rearranged: Answer: | |
3D structure
3D structure
Basic properties
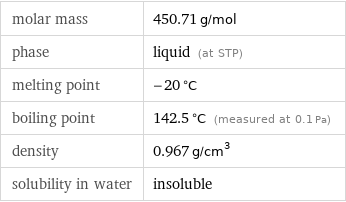
molar mass | 450.71 g/mol phase | liquid (at STP) melting point | -20 °C boiling point | 142.5 °C (measured at 0.1 Pa) density | 0.967 g/cm^3 solubility in water | insoluble
Units

Hydrophobicity and permeability properties

experimental LogP hydrophobicity | 9.3 predicted LogP hydrophobicity | 8.48 predicted LogS | -6.88
Basic drug properties

approval status | approved | small molecule drug categories | antifibrinolytic agent | vitamin | vitamin (Vitamin K) dosage forms | intramuscular: injection, solution | intravenous: injection, solution | subcutaneous: injection, solution

brand names | aqua-mephytin | aquaMEPHYTON | combinal K1 | K-ject | kativ N | kephton | kinadion | konakion | mephyton | mono-kay | monodion | synthex P
Liquid properties (at STP)
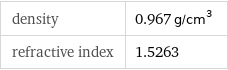
density | 0.967 g/cm^3 refractive index | 1.5263
Units

Chemical identifiers
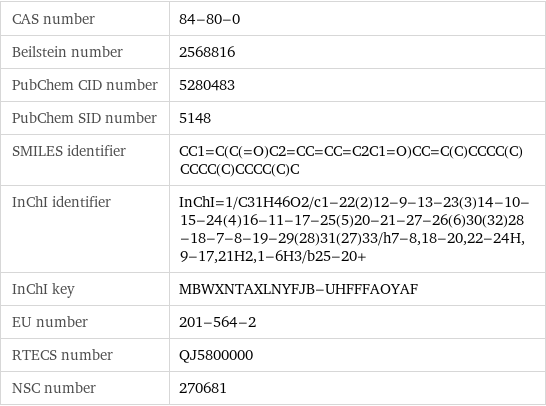
CAS number | 84-80-0 Beilstein number | 2568816 PubChem CID number | 5280483 PubChem SID number | 5148 SMILES identifier | CC1=C(C(=O)C2=CC=CC=C2C1=O)CC=C(C)CCCC(C)CCCC(C)CCCC(C)C InChI identifier | InChI=1/C31H46O2/c1-22(2)12-9-13-23(3)14-10-15-24(4)16-11-17-25(5)20-21-27-26(6)30(32)28-18-7-8-19-29(28)31(27)33/h7-8, 18-20, 22-24H, 9-17, 21H2, 1-6H3/b25-20+ InChI key | MBWXNTAXLNYFJB-UHFFFAOYAF EU number | 201-564-2 RTECS number | QJ5800000 NSC number | 270681
NFPA label

NFPA label

NFPA health rating | 1 NFPA fire rating | 0 NFPA reactivity rating | 0
Safety properties

flash point | 110 °C

DOT hazard class | 3 DOT numbers | 1224
Toxicity properties

odor | odorless

RTECS classes | tumorigen | drug | human data