Input interpretation
hydrogen (chemical element) | oxygen (chemical element) | iodine (chemical element)
Periodic table location
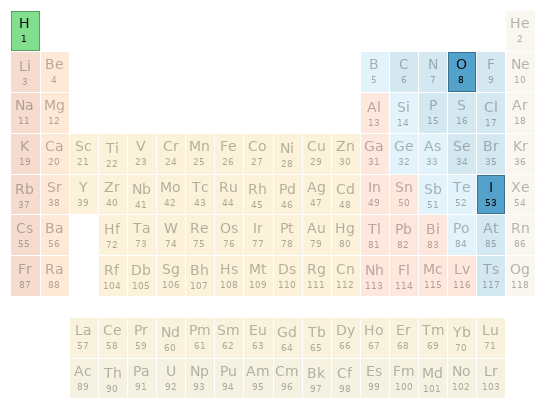
Periodic table location
Images
Images
Basic elemental properties
![| hydrogen | oxygen | iodine atomic symbol | H | O | I atomic number | 1 | 8 | 53 short electronic configuration | 1s^1 | [He]2s^22p^4 | [Kr]5s^24d^105p^5 Aufbau diagram | 1s | 2p 2s | 5p 4d 5s block | s | p | p group | 1 | 16 | 17 period | 1 | 2 | 5 atomic mass | 1.008 u | 15.999 u | 126.90447 u half-life | (stable) | (stable) | (stable)](../image_source/542674f7243f076fae997df070d9b4ef.png)
| hydrogen | oxygen | iodine atomic symbol | H | O | I atomic number | 1 | 8 | 53 short electronic configuration | 1s^1 | [He]2s^22p^4 | [Kr]5s^24d^105p^5 Aufbau diagram | 1s | 2p 2s | 5p 4d 5s block | s | p | p group | 1 | 16 | 17 period | 1 | 2 | 5 atomic mass | 1.008 u | 15.999 u | 126.90447 u half-life | (stable) | (stable) | (stable)
Thermodynamic properties
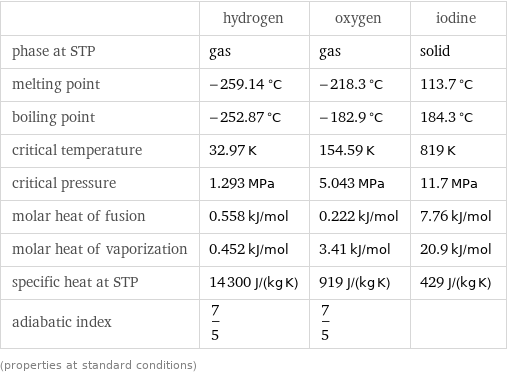
| hydrogen | oxygen | iodine phase at STP | gas | gas | solid melting point | -259.14 °C | -218.3 °C | 113.7 °C boiling point | -252.87 °C | -182.9 °C | 184.3 °C critical temperature | 32.97 K | 154.59 K | 819 K critical pressure | 1.293 MPa | 5.043 MPa | 11.7 MPa molar heat of fusion | 0.558 kJ/mol | 0.222 kJ/mol | 7.76 kJ/mol molar heat of vaporization | 0.452 kJ/mol | 3.41 kJ/mol | 20.9 kJ/mol specific heat at STP | 14300 J/(kg K) | 919 J/(kg K) | 429 J/(kg K) adiabatic index | 7/5 | 7/5 | (properties at standard conditions)
Material properties
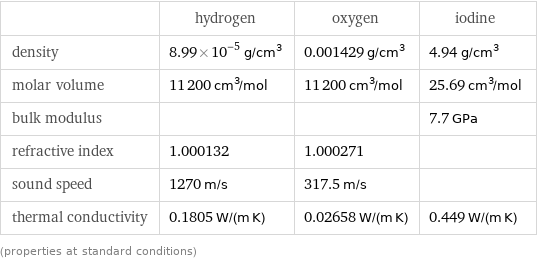
| hydrogen | oxygen | iodine density | 8.99×10^-5 g/cm^3 | 0.001429 g/cm^3 | 4.94 g/cm^3 molar volume | 11200 cm^3/mol | 11200 cm^3/mol | 25.69 cm^3/mol bulk modulus | | | 7.7 GPa refractive index | 1.000132 | 1.000271 | sound speed | 1270 m/s | 317.5 m/s | thermal conductivity | 0.1805 W/(m K) | 0.02658 W/(m K) | 0.449 W/(m K) (properties at standard conditions)
Electromagnetic properties
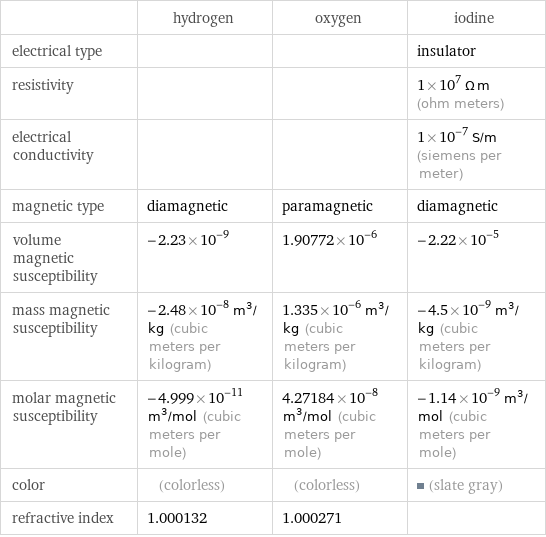
| hydrogen | oxygen | iodine electrical type | | | insulator resistivity | | | 1×10^7 Ω m (ohm meters) electrical conductivity | | | 1×10^-7 S/m (siemens per meter) magnetic type | diamagnetic | paramagnetic | diamagnetic volume magnetic susceptibility | -2.23×10^-9 | 1.90772×10^-6 | -2.22×10^-5 mass magnetic susceptibility | -2.48×10^-8 m^3/kg (cubic meters per kilogram) | 1.335×10^-6 m^3/kg (cubic meters per kilogram) | -4.5×10^-9 m^3/kg (cubic meters per kilogram) molar magnetic susceptibility | -4.999×10^-11 m^3/mol (cubic meters per mole) | 4.27184×10^-8 m^3/mol (cubic meters per mole) | -1.14×10^-9 m^3/mol (cubic meters per mole) color | (colorless) | (colorless) | (slate gray) refractive index | 1.000132 | 1.000271 |
Reactivity
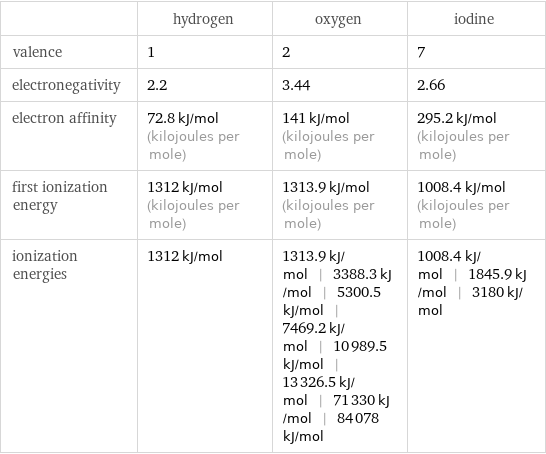
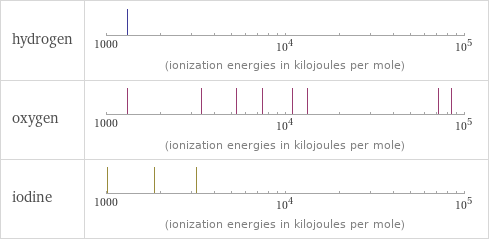
| hydrogen | oxygen | iodine valence | 1 | 2 | 7 electronegativity | 2.2 | 3.44 | 2.66 electron affinity | 72.8 kJ/mol (kilojoules per mole) | 141 kJ/mol (kilojoules per mole) | 295.2 kJ/mol (kilojoules per mole) first ionization energy | 1312 kJ/mol (kilojoules per mole) | 1313.9 kJ/mol (kilojoules per mole) | 1008.4 kJ/mol (kilojoules per mole) ionization energies | 1312 kJ/mol | 1313.9 kJ/mol | 3388.3 kJ/mol | 5300.5 kJ/mol | 7469.2 kJ/mol | 10989.5 kJ/mol | 13326.5 kJ/mol | 71330 kJ/mol | 84078 kJ/mol | 1008.4 kJ/mol | 1845.9 kJ/mol | 3180 kJ/mol
Reactivity
Atomic properties
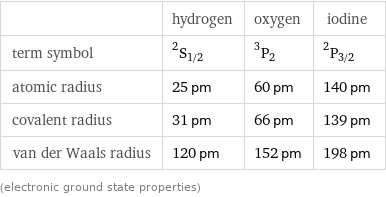
| hydrogen | oxygen | iodine term symbol | ^2S_(1/2) | ^3P_2 | ^2P_(3/2) atomic radius | 25 pm | 60 pm | 140 pm covalent radius | 31 pm | 66 pm | 139 pm van der Waals radius | 120 pm | 152 pm | 198 pm (electronic ground state properties)
Abundances
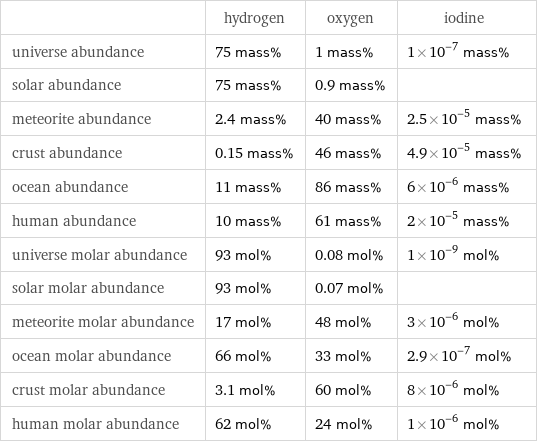
| hydrogen | oxygen | iodine universe abundance | 75 mass% | 1 mass% | 1×10^-7 mass% solar abundance | 75 mass% | 0.9 mass% | meteorite abundance | 2.4 mass% | 40 mass% | 2.5×10^-5 mass% crust abundance | 0.15 mass% | 46 mass% | 4.9×10^-5 mass% ocean abundance | 11 mass% | 86 mass% | 6×10^-6 mass% human abundance | 10 mass% | 61 mass% | 2×10^-5 mass% universe molar abundance | 93 mol% | 0.08 mol% | 1×10^-9 mol% solar molar abundance | 93 mol% | 0.07 mol% | meteorite molar abundance | 17 mol% | 48 mol% | 3×10^-6 mol% ocean molar abundance | 66 mol% | 33 mol% | 2.9×10^-7 mol% crust molar abundance | 3.1 mol% | 60 mol% | 8×10^-6 mol% human molar abundance | 62 mol% | 24 mol% | 1×10^-6 mol%
Nuclear properties
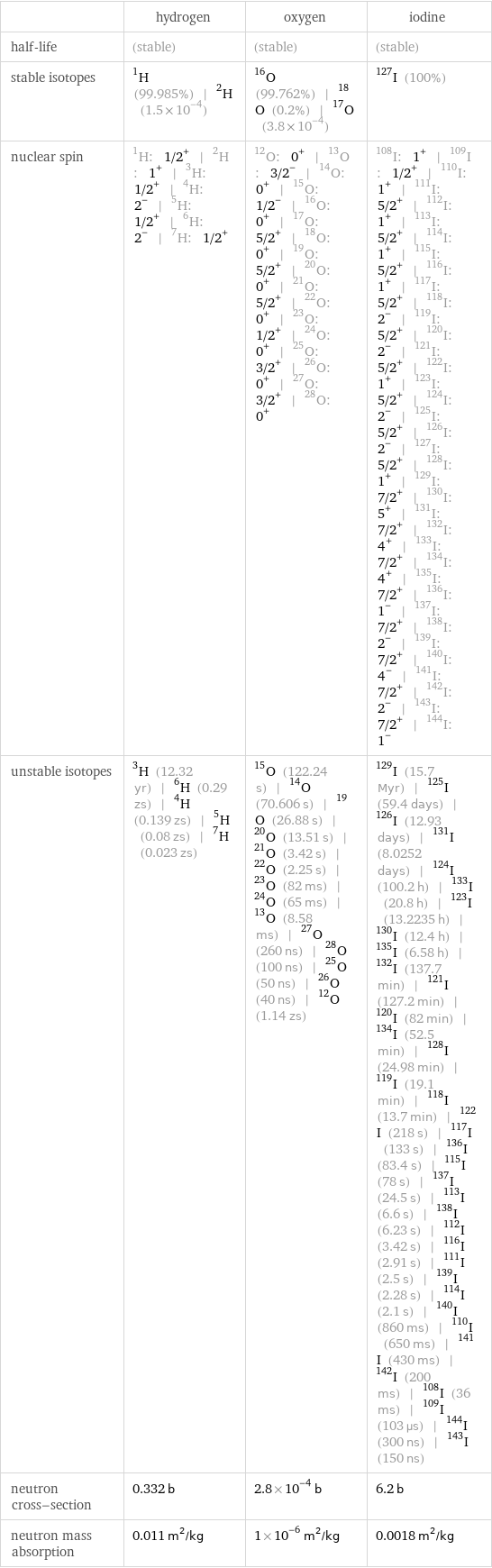
| hydrogen | oxygen | iodine half-life | (stable) | (stable) | (stable) stable isotopes | H-1 (99.985%) | H-2 (1.5×10^-4) | O-16 (99.762%) | O-18 (0.2%) | O-17 (3.8×10^-4) | I-127 (100%) nuclear spin | H-1: 1/2^+ | H-2: 1^+ | H-3: 1/2^+ | H-4: 2^- | H-5: 1/2^+ | H-6: 2^- | H-7: 1/2^+ | O-12: 0^+ | O-13: 3/2^- | O-14: 0^+ | O-15: 1/2^- | O-16: 0^+ | O-17: 5/2^+ | O-18: 0^+ | O-19: 5/2^+ | O-20: 0^+ | O-21: 5/2^+ | O-22: 0^+ | O-23: 1/2^+ | O-24: 0^+ | O-25: 3/2^+ | O-26: 0^+ | O-27: 3/2^+ | O-28: 0^+ | I-108: 1^+ | I-109: 1/2^+ | I-110: 1^+ | I-111: 5/2^+ | I-112: 1^+ | I-113: 5/2^+ | I-114: 1^+ | I-115: 5/2^+ | I-116: 1^+ | I-117: 5/2^+ | I-118: 2^- | I-119: 5/2^+ | I-120: 2^- | I-121: 5/2^+ | I-122: 1^+ | I-123: 5/2^+ | I-124: 2^- | I-125: 5/2^+ | I-126: 2^- | I-127: 5/2^+ | I-128: 1^+ | I-129: 7/2^+ | I-130: 5^+ | I-131: 7/2^+ | I-132: 4^+ | I-133: 7/2^+ | I-134: 4^+ | I-135: 7/2^+ | I-136: 1^- | I-137: 7/2^+ | I-138: 2^- | I-139: 7/2^+ | I-140: 4^- | I-141: 7/2^+ | I-142: 2^- | I-143: 7/2^+ | I-144: 1^- unstable isotopes | H-3 (12.32 yr) | H-6 (0.29 zs) | H-4 (0.139 zs) | H-5 (0.08 zs) | H-7 (0.023 zs) | O-15 (122.24 s) | O-14 (70.606 s) | O-19 (26.88 s) | O-20 (13.51 s) | O-21 (3.42 s) | O-22 (2.25 s) | O-23 (82 ms) | O-24 (65 ms) | O-13 (8.58 ms) | O-27 (260 ns) | O-28 (100 ns) | O-25 (50 ns) | O-26 (40 ns) | O-12 (1.14 zs) | I-129 (15.7 Myr) | I-125 (59.4 days) | I-126 (12.93 days) | I-131 (8.0252 days) | I-124 (100.2 h) | I-133 (20.8 h) | I-123 (13.2235 h) | I-130 (12.4 h) | I-135 (6.58 h) | I-132 (137.7 min) | I-121 (127.2 min) | I-120 (82 min) | I-134 (52.5 min) | I-128 (24.98 min) | I-119 (19.1 min) | I-118 (13.7 min) | I-122 (218 s) | I-117 (133 s) | I-136 (83.4 s) | I-115 (78 s) | I-137 (24.5 s) | I-113 (6.6 s) | I-138 (6.23 s) | I-112 (3.42 s) | I-116 (2.91 s) | I-111 (2.5 s) | I-139 (2.28 s) | I-114 (2.1 s) | I-140 (860 ms) | I-110 (650 ms) | I-141 (430 ms) | I-142 (200 ms) | I-108 (36 ms) | I-109 (103 µs) | I-144 (300 ns) | I-143 (150 ns) neutron cross-section | 0.332 b | 2.8×10^-4 b | 6.2 b neutron mass absorption | 0.011 m^2/kg | 1×10^-6 m^2/kg | 0.0018 m^2/kg