Input interpretation
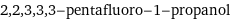
2, 2, 3, 3, 3-pentafluoro-1-propanol
Chemical names and formulas
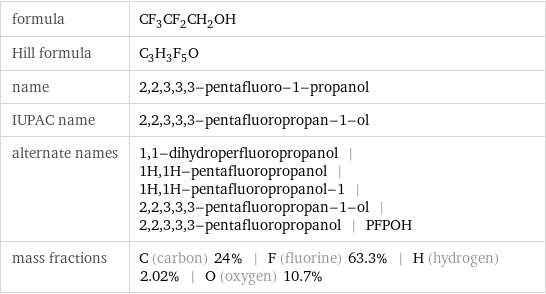
formula | CF_3CF_2CH_2OH Hill formula | C_3H_3F_5O name | 2, 2, 3, 3, 3-pentafluoro-1-propanol IUPAC name | 2, 2, 3, 3, 3-pentafluoropropan-1-ol alternate names | 1, 1-dihydroperfluoropropanol | 1H, 1H-pentafluoropropanol | 1H, 1H-pentafluoropropanol-1 | 2, 2, 3, 3, 3-pentafluoropropan-1-ol | 2, 2, 3, 3, 3-pentafluoropropanol | PFPOH mass fractions | C (carbon) 24% | F (fluorine) 63.3% | H (hydrogen) 2.02% | O (oxygen) 10.7%
Lewis structure
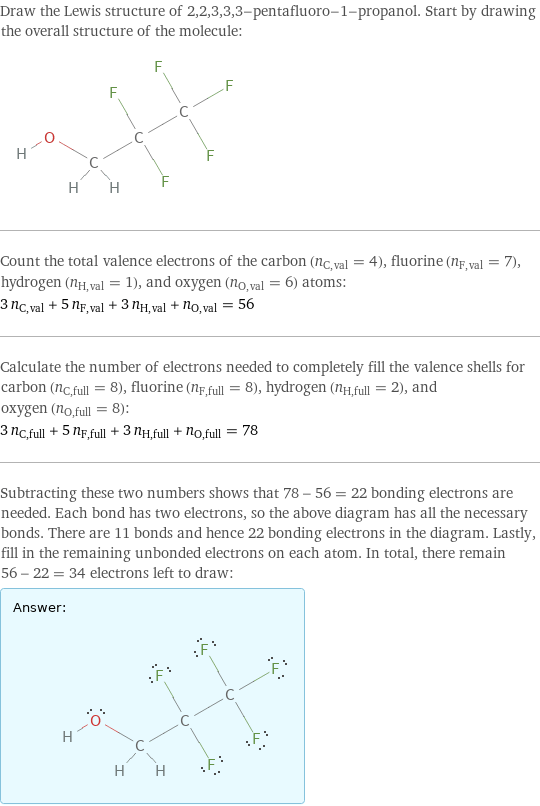
Draw the Lewis structure of 2, 2, 3, 3, 3-pentafluoro-1-propanol. Start by drawing the overall structure of the molecule: Count the total valence electrons of the carbon (n_C, val = 4), fluorine (n_F, val = 7), hydrogen (n_H, val = 1), and oxygen (n_O, val = 6) atoms: 3 n_C, val + 5 n_F, val + 3 n_H, val + n_O, val = 56 Calculate the number of electrons needed to completely fill the valence shells for carbon (n_C, full = 8), fluorine (n_F, full = 8), hydrogen (n_H, full = 2), and oxygen (n_O, full = 8): 3 n_C, full + 5 n_F, full + 3 n_H, full + n_O, full = 78 Subtracting these two numbers shows that 78 - 56 = 22 bonding electrons are needed. Each bond has two electrons, so the above diagram has all the necessary bonds. There are 11 bonds and hence 22 bonding electrons in the diagram. Lastly, fill in the remaining unbonded electrons on each atom. In total, there remain 56 - 22 = 34 electrons left to draw: Answer: | |
3D structure
3D structure
Basic properties

molar mass | 150.05 g/mol phase | liquid (at STP) boiling point | 80 °C (measured at 99708 Pa) density | 1.505 g/cm^3
Units

Liquid properties (at STP)
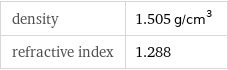
density | 1.505 g/cm^3 refractive index | 1.288
Units

Chemical identifiers
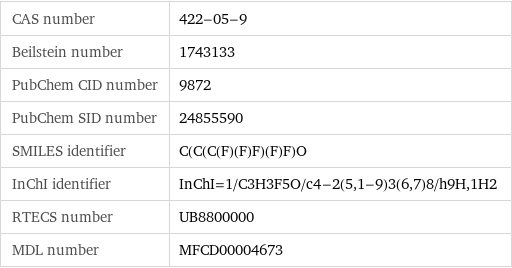
CAS number | 422-05-9 Beilstein number | 1743133 PubChem CID number | 9872 PubChem SID number | 24855590 SMILES identifier | C(C(C(F)(F)F)(F)F)O InChI identifier | InChI=1/C3H3F5O/c4-2(5, 1-9)3(6, 7)8/h9H, 1H2 RTECS number | UB8800000 MDL number | MFCD00004673
NFPA label

NFPA label

NFPA health rating | 2 NFPA fire rating | 0 NFPA reactivity rating | 0
Toxicity properties

RTECS classes | other