Input interpretation
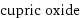
cupric oxide
Chemical names and formulas

formula | CuO name | cupric oxide alternate names | copper brown | copper(II) oxide | copper monooxide | copper monoxide | copper oxide | ketocopper | oxocopper mass fractions | Cu (copper) 79.9% | O (oxygen) 20.1%
Structure diagram
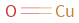
Structure diagram
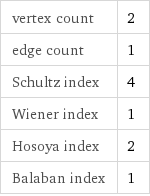
vertex count | 2 edge count | 1 Schultz index | 4 Wiener index | 1 Hosoya index | 2 Balaban index | 1
Basic properties
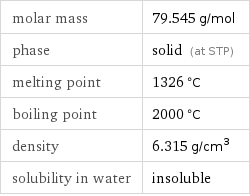
molar mass | 79.545 g/mol phase | solid (at STP) melting point | 1326 °C boiling point | 2000 °C density | 6.315 g/cm^3 solubility in water | insoluble
Units

Solid properties (at STP)

density | 6.315 g/cm^3 vapor pressure | 1×10^-4 mmHg (at 800 °C)
Units

Thermodynamic properties

specific heat capacity c_p | solid | 0.5318 J/(g K) molar heat capacity c_p | solid | 42.3 J/(mol K) specific free energy of formation Δ_fG° | solid | -1.631 kJ/g molar free energy of formation Δ_fG° | solid | -129.7 kJ/mol specific heat of formation Δ_fH° | solid | -1.977 kJ/g molar heat of formation Δ_fH° | solid | -157.3 kJ/mol specific entropy S° | solid | 0.5406 J/(g K) molar entropy S° | solid | 43 J/(mol K) molar heat of fusion | 49 kJ/mol | specific heat of fusion | 0.62 kJ/g | (at STP)
Chemical identifiers
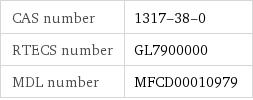
CAS number | 1317-38-0 RTECS number | GL7900000 MDL number | MFCD00010979
NFPA label

NFPA label

NFPA health rating | 2 NFPA fire rating | 0 NFPA reactivity rating | 0
Toxicity properties

RTECS classes | other