Input interpretation
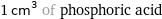
1 cm^3 of phosphoric acid
Basic properties for 1 cm^3
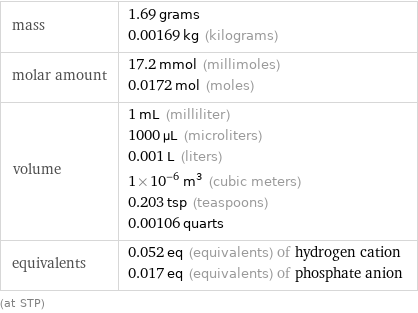
mass | 1.69 grams 0.00169 kg (kilograms) molar amount | 17.2 mmol (millimoles) 0.0172 mol (moles) volume | 1 mL (milliliter) 1000 µL (microliters) 0.001 L (liters) 1×10^-6 m^3 (cubic meters) 0.203 tsp (teaspoons) 0.00106 quarts equivalents | 0.052 eq (equivalents) of hydrogen cation 0.017 eq (equivalents) of phosphate anion (at STP)
Corresponding quantities

sphere radius | 6.204 mm (millimeters) side of a cube | 10 mm (millimeters)
Thermodynamic properties for 1 cm^3
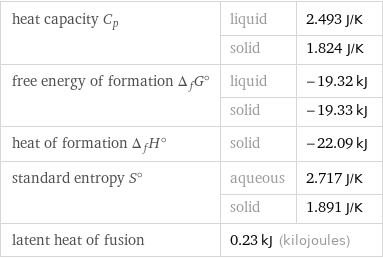
heat capacity C_p | liquid | 2.493 J/K | solid | 1.824 J/K free energy of formation Δ_fG° | liquid | -19.32 kJ | solid | -19.33 kJ heat of formation Δ_fH° | solid | -22.09 kJ standard entropy S° | aqueous | 2.717 J/K | solid | 1.891 J/K latent heat of fusion | 0.23 kJ (kilojoules) |
Units
Energy vs. temperature for 1 cm^3
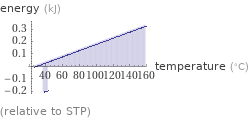
(relative to STP)
Units

Phase change energies for 1 cm^3 from 25 °C

energy required to heat to boiling point | 0.332 kJ (kilojoules) energy released from cooling to freezing point | -0.0434 kJ (kilojoules) energy released from converting to solid | 0.23 kJ (kilojoules) energy released from cooling to freezing point and converting to solid | 0.187 kJ (kilojoules)
Mass composition for 1 cm^3
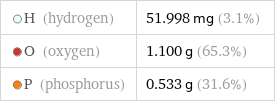
H (hydrogen) | 51.998 mg (3.1%) O (oxygen) | 1.100 g (65.3%) P (phosphorus) | 0.533 g (31.6%)
Mass composition for 1 cm^3
Lewis structure
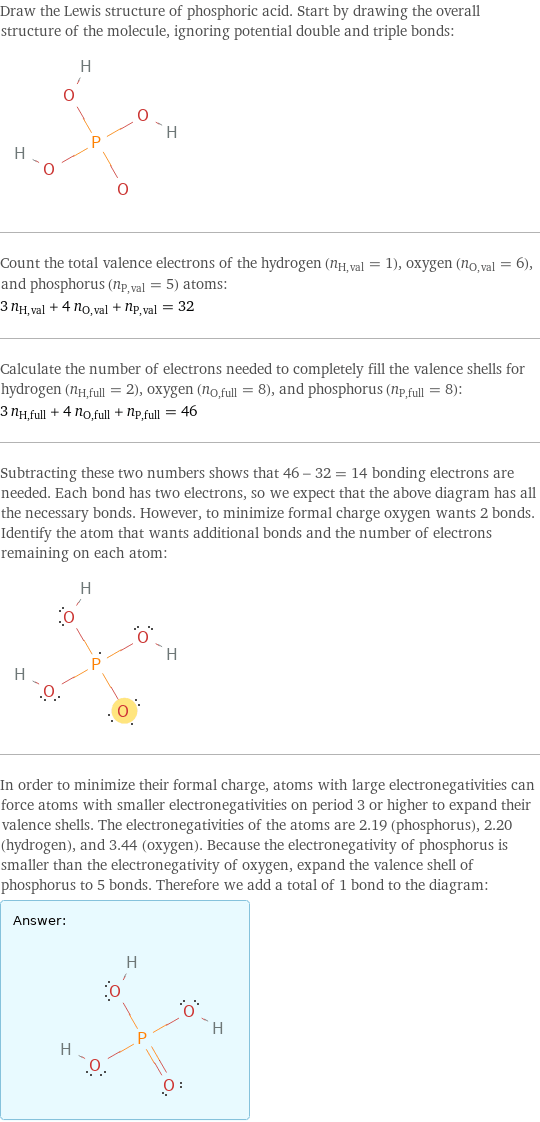
Draw the Lewis structure of phosphoric acid. Start by drawing the overall structure of the molecule, ignoring potential double and triple bonds: Count the total valence electrons of the hydrogen (n_H, val = 1), oxygen (n_O, val = 6), and phosphorus (n_P, val = 5) atoms: 3 n_H, val + 4 n_O, val + n_P, val = 32 Calculate the number of electrons needed to completely fill the valence shells for hydrogen (n_H, full = 2), oxygen (n_O, full = 8), and phosphorus (n_P, full = 8): 3 n_H, full + 4 n_O, full + n_P, full = 46 Subtracting these two numbers shows that 46 - 32 = 14 bonding electrons are needed. Each bond has two electrons, so we expect that the above diagram has all the necessary bonds. However, to minimize formal charge oxygen wants 2 bonds. Identify the atom that wants additional bonds and the number of electrons remaining on each atom: In order to minimize their formal charge, atoms with large electronegativities can force atoms with smaller electronegativities on period 3 or higher to expand their valence shells. The electronegativities of the atoms are 2.19 (phosphorus), 2.20 (hydrogen), and 3.44 (oxygen). Because the electronegativity of phosphorus is smaller than the electronegativity of oxygen, expand the valence shell of phosphorus to 5 bonds. Therefore we add a total of 1 bond to the diagram: Answer: | |
Chemical names and formulas
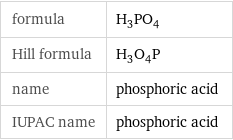
formula | H_3PO_4 Hill formula | H_3O_4P name | phosphoric acid IUPAC name | phosphoric acid
Substance properties
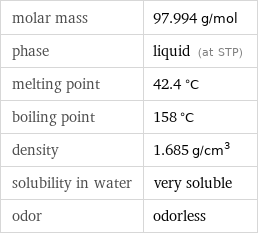
molar mass | 97.994 g/mol phase | liquid (at STP) melting point | 42.4 °C boiling point | 158 °C density | 1.685 g/cm^3 solubility in water | very soluble odor | odorless
Units
