Input interpretation
barium fluoride
Chemical names and formulas

formula | BaF_2 name | barium fluoride IUPAC name | barium(+2) cation difluoride alternate names | barium(+2) cation difluoride | barium difluoride mass fractions | Ba (barium) 78.3% | F (fluorine) 21.7%
Structure diagram
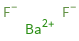
Structure diagram
Basic properties
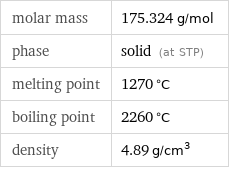
molar mass | 175.324 g/mol phase | solid (at STP) melting point | 1270 °C boiling point | 2260 °C density | 4.89 g/cm^3
Units

Solid properties (at STP)
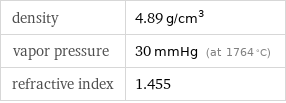
density | 4.89 g/cm^3 vapor pressure | 30 mmHg (at 1764 °C) refractive index | 1.455
Units

Thermodynamic properties
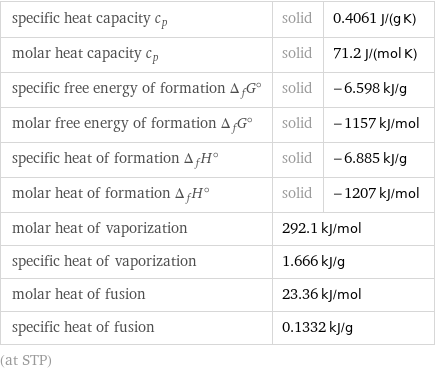
specific heat capacity c_p | solid | 0.4061 J/(g K) molar heat capacity c_p | solid | 71.2 J/(mol K) specific free energy of formation Δ_fG° | solid | -6.598 kJ/g molar free energy of formation Δ_fG° | solid | -1157 kJ/mol specific heat of formation Δ_fH° | solid | -6.885 kJ/g molar heat of formation Δ_fH° | solid | -1207 kJ/mol molar heat of vaporization | 292.1 kJ/mol | specific heat of vaporization | 1.666 kJ/g | molar heat of fusion | 23.36 kJ/mol | specific heat of fusion | 0.1332 kJ/g | (at STP)
Chemical identifiers
![CAS number | 7787-32-8 PubChem CID number | 62670 PubChem SID number | 24852114 SMILES identifier | [F-].[F-].[Ba+2] InChI identifier | InChI=1/Ba.2FH/h;2*1H/q+2;;/p-2/fBa.2F/h;2*1h/qm;2*-1 RTECS number | CQ9100000 MDL number | MFCD00003450](../image_source/9afaa189ec8df2cff9935748230e419b.png)
CAS number | 7787-32-8 PubChem CID number | 62670 PubChem SID number | 24852114 SMILES identifier | [F-].[F-].[Ba+2] InChI identifier | InChI=1/Ba.2FH/h;2*1H/q+2;;/p-2/fBa.2F/h;2*1h/qm;2*-1 RTECS number | CQ9100000 MDL number | MFCD00003450
NFPA label

NFPA label

NFPA health rating | 2 NFPA fire rating | 0 NFPA reactivity rating | 0
Toxicity properties

RTECS classes | reproductive effector
Ion equivalents

Ba^(2+) (barium cation) | 1 F^- (fluoride anion) | 2