Input interpretation
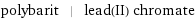
polybarit | lead(II) chromate
Chemical names and formulas
![| polybarit | lead(II) chromate formula | BaH_2S | PbCrO_4 Hill formula | BaH_2S | CrO_4Pb name | polybarit | lead(II) chromate IUPAC name | | plumbous dioxido-dioxochromium alternate names | barium polysulfide | barium polysulfides | bariumpolysulfid [German] | chrome yellow | chromic acid, lead(II) salt (1:1) | lead chromate | plumbous chromate | plumbous dioxido-dioxo-chromium mass fractions | ([[All, 1]] ({, hydrogen | helium | lithium | beryllium | boron | carbon | nitrogen | oxygen | fluorine | neon | sodium | magnesium | aluminum | silicon | phosphorus | sulfur | chlorine | argon | potassium | calcium | scandium | titanium | vanadium | chromium | manganese | iron | cobalt | nickel | copper | zinc | gallium | germanium | arsenic | selenium | bromine | krypton | rubidium | strontium | yttrium | zirconium | niobium | molybdenum | technetium | ruthenium | rhodium | palladium | silver | cadmium | indium | tin | antimony | tellurium | iodine | xenon | cesium | barium | lanthanum | cerium | praseodymium | neodymium | promethium | samarium | europium | gadolinium | terbium | dysprosium | holmium | erbium | thulium | ytterbium | lutetium | hafnium | tantalum | tungsten | rhenium | osmium | iridium | platinum | gold | mercury | thallium | lead | bismuth | polonium | astatine | radon | francium | radium | actinium | thorium | protactinium | uranium | neptunium | plutonium | americium | curium | berkelium | californium | einsteinium | fermium | mendelevium | nobelium | lawrencium | rutherfordium | dubnium | seaborgium | bohrium | hassium | meitnerium | darmstadtium | roentgenium | copernicium | nihonium | flerovium | moscovium | livermorium | tennessine | oganesson, hydrogen})->[[All, 2]] g/100 g (grams per hundred grams))^T | Cr (chromium) 16.1% | O (oxygen) 19.8% | Pb (lead) 64.1%](../image_source/ae72d9f3da0ec8f532fd99c92341e4de.png)
| polybarit | lead(II) chromate formula | BaH_2S | PbCrO_4 Hill formula | BaH_2S | CrO_4Pb name | polybarit | lead(II) chromate IUPAC name | | plumbous dioxido-dioxochromium alternate names | barium polysulfide | barium polysulfides | bariumpolysulfid [German] | chrome yellow | chromic acid, lead(II) salt (1:1) | lead chromate | plumbous chromate | plumbous dioxido-dioxo-chromium mass fractions | ([[All, 1]] ({, hydrogen | helium | lithium | beryllium | boron | carbon | nitrogen | oxygen | fluorine | neon | sodium | magnesium | aluminum | silicon | phosphorus | sulfur | chlorine | argon | potassium | calcium | scandium | titanium | vanadium | chromium | manganese | iron | cobalt | nickel | copper | zinc | gallium | germanium | arsenic | selenium | bromine | krypton | rubidium | strontium | yttrium | zirconium | niobium | molybdenum | technetium | ruthenium | rhodium | palladium | silver | cadmium | indium | tin | antimony | tellurium | iodine | xenon | cesium | barium | lanthanum | cerium | praseodymium | neodymium | promethium | samarium | europium | gadolinium | terbium | dysprosium | holmium | erbium | thulium | ytterbium | lutetium | hafnium | tantalum | tungsten | rhenium | osmium | iridium | platinum | gold | mercury | thallium | lead | bismuth | polonium | astatine | radon | francium | radium | actinium | thorium | protactinium | uranium | neptunium | plutonium | americium | curium | berkelium | californium | einsteinium | fermium | mendelevium | nobelium | lawrencium | rutherfordium | dubnium | seaborgium | bohrium | hassium | meitnerium | darmstadtium | roentgenium | copernicium | nihonium | flerovium | moscovium | livermorium | tennessine | oganesson, hydrogen})->[[All, 2]] g/100 g (grams per hundred grams))^T | Cr (chromium) 16.1% | O (oxygen) 19.8% | Pb (lead) 64.1%
Structure diagrams
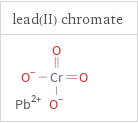
Structure diagrams
Basic properties
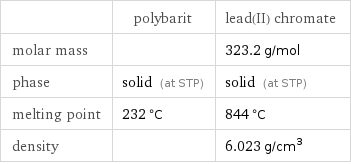
| polybarit | lead(II) chromate molar mass | | 323.2 g/mol phase | solid (at STP) | solid (at STP) melting point | 232 °C | 844 °C density | | 6.023 g/cm^3
Units
