Input interpretation
vanadium(V) oxychloride
Chemical names and formulas
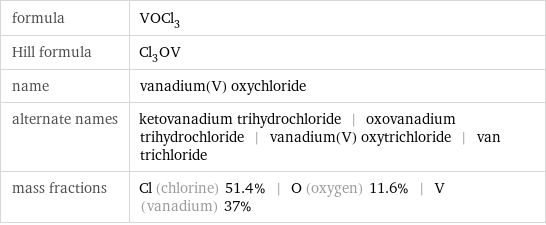
formula | VOCl_3 Hill formula | Cl_3OV name | vanadium(V) oxychloride alternate names | ketovanadium trihydrochloride | oxovanadium trihydrochloride | vanadium(V) oxytrichloride | van trichloride mass fractions | Cl (chlorine) 51.4% | O (oxygen) 11.6% | V (vanadium) 37%
Structure diagram
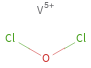
Structure diagram
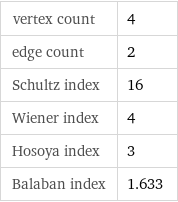
vertex count | 4 edge count | 2 Schultz index | 16 Wiener index | 4 Hosoya index | 3 Balaban index | 1.633
Basic properties
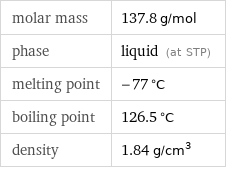
molar mass | 137.8 g/mol phase | liquid (at STP) melting point | -77 °C boiling point | 126.5 °C density | 1.84 g/cm^3
Units

Liquid properties (at STP)
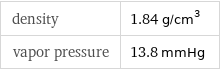
density | 1.84 g/cm^3 vapor pressure | 13.8 mmHg
Units

Thermodynamic properties
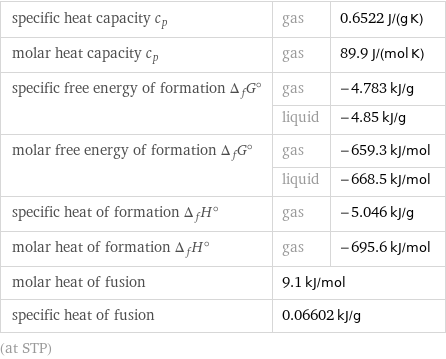
specific heat capacity c_p | gas | 0.6522 J/(g K) molar heat capacity c_p | gas | 89.9 J/(mol K) specific free energy of formation Δ_fG° | gas | -4.783 kJ/g | liquid | -4.85 kJ/g molar free energy of formation Δ_fG° | gas | -659.3 kJ/mol | liquid | -668.5 kJ/mol specific heat of formation Δ_fH° | gas | -5.046 kJ/g molar heat of formation Δ_fH° | gas | -695.6 kJ/mol molar heat of fusion | 9.1 kJ/mol | specific heat of fusion | 0.06602 kJ/g | (at STP)
Chemical identifiers
![CAS number | 7727-18-6 SMILES identifier | ClOCl.[V+5] RTECS number | YW2975000 MDL number | MFCD00011459](../image_source/13cf12a37bee5f743998b29772b83d15.png)
CAS number | 7727-18-6 SMILES identifier | ClOCl.[V+5] RTECS number | YW2975000 MDL number | MFCD00011459