Input interpretation
monoprotic acids
Members

trifluoromethanesulfonic acid | perchloric acid | hydrobromic acid | hydroiodic acid | hydrochloric acid | methanesulfonic acid | nitric acid | isothiocyanic acid | trifluoroacetic acid | iodic acid | fluoroacetic acid | chloroacetic acid | hydrofluoric acid | formic acid | benzoic acid | acetic acid | hypochlorous acid | phenol | hydrogen cyanide | ethanol (total: 20)
Basic properties
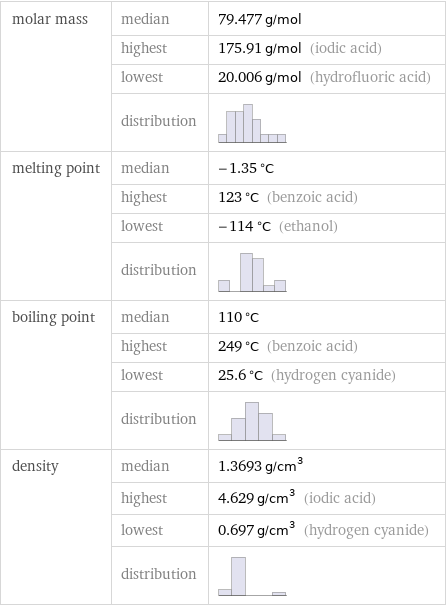
molar mass | median | 79.477 g/mol | highest | 175.91 g/mol (iodic acid) | lowest | 20.006 g/mol (hydrofluoric acid) | distribution | melting point | median | -1.35 °C | highest | 123 °C (benzoic acid) | lowest | -114 °C (ethanol) | distribution | boiling point | median | 110 °C | highest | 249 °C (benzoic acid) | lowest | 25.6 °C (hydrogen cyanide) | distribution | density | median | 1.3693 g/cm^3 | highest | 4.629 g/cm^3 (iodic acid) | lowest | 0.697 g/cm^3 (hydrogen cyanide) | distribution |
Units
