Input interpretation
diamagnetic elements
Members

antimony | argon | arsenic | beryllium | bismuth | boron | bromine | cadmium | carbon | chlorine | copper | gallium | germanium | gold | helium | hydrogen | indium | iodine | krypton | lead | mercury | neon | nitrogen | phosphorus | selenium | silicon | silver | sulfur | tellurium | thallium | tin | xenon | zinc (total: 33)
Periodic table location
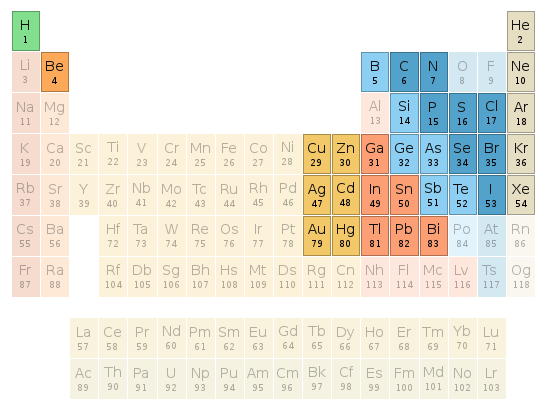
Periodic table location
Images
Images
Basic elemental properties
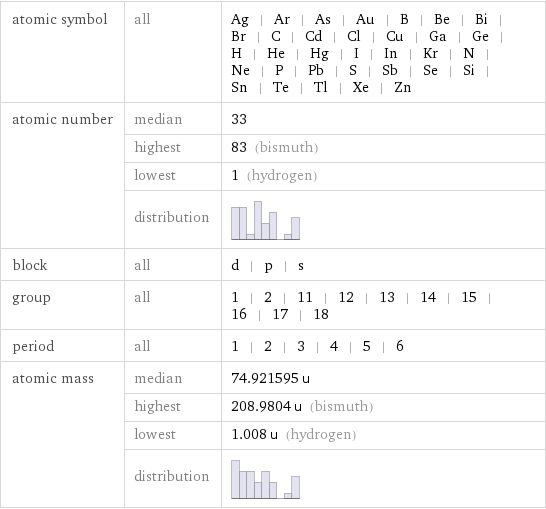
atomic symbol | all | Ag | Ar | As | Au | B | Be | Bi | Br | C | Cd | Cl | Cu | Ga | Ge | H | He | Hg | I | In | Kr | N | Ne | P | Pb | S | Sb | Se | Si | Sn | Te | Tl | Xe | Zn atomic number | median | 33 | highest | 83 (bismuth) | lowest | 1 (hydrogen) | distribution | block | all | d | p | s group | all | 1 | 2 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 period | all | 1 | 2 | 3 | 4 | 5 | 6 atomic mass | median | 74.921595 u | highest | 208.9804 u (bismuth) | lowest | 1.008 u (hydrogen) | distribution |
Thermodynamic properties
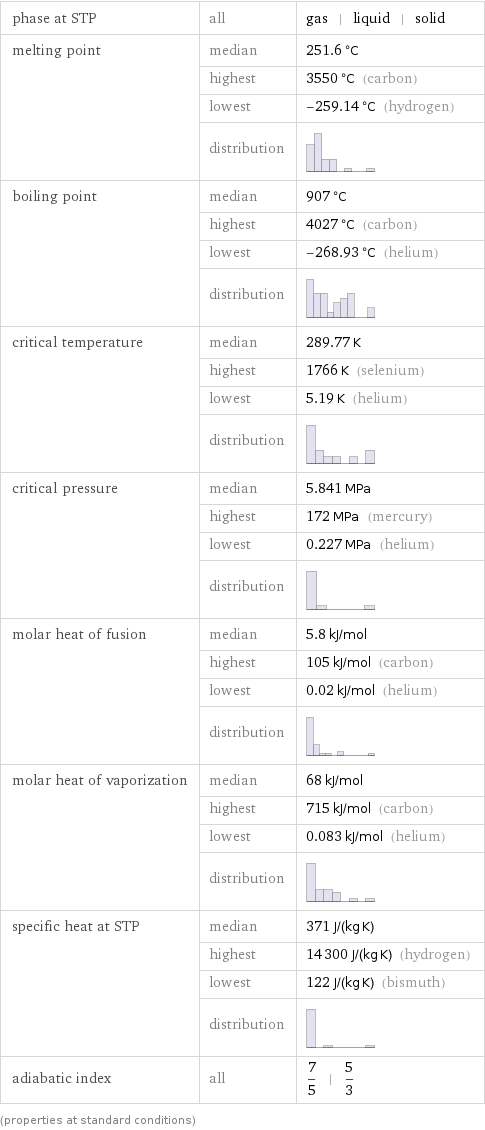
phase at STP | all | gas | liquid | solid melting point | median | 251.6 °C | highest | 3550 °C (carbon) | lowest | -259.14 °C (hydrogen) | distribution | boiling point | median | 907 °C | highest | 4027 °C (carbon) | lowest | -268.93 °C (helium) | distribution | critical temperature | median | 289.77 K | highest | 1766 K (selenium) | lowest | 5.19 K (helium) | distribution | critical pressure | median | 5.841 MPa | highest | 172 MPa (mercury) | lowest | 0.227 MPa (helium) | distribution | molar heat of fusion | median | 5.8 kJ/mol | highest | 105 kJ/mol (carbon) | lowest | 0.02 kJ/mol (helium) | distribution | molar heat of vaporization | median | 68 kJ/mol | highest | 715 kJ/mol (carbon) | lowest | 0.083 kJ/mol (helium) | distribution | specific heat at STP | median | 371 J/(kg K) | highest | 14300 J/(kg K) (hydrogen) | lowest | 122 J/(kg K) (bismuth) | distribution | adiabatic index | all | 7/5 | 5/3 (properties at standard conditions)
Material properties
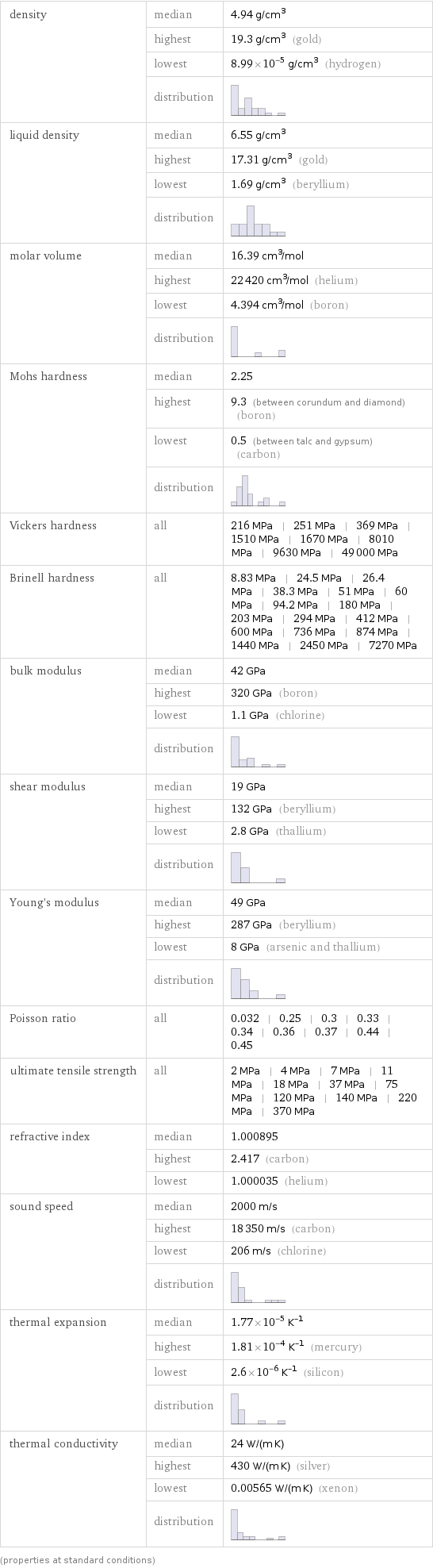
density | median | 4.94 g/cm^3 | highest | 19.3 g/cm^3 (gold) | lowest | 8.99×10^-5 g/cm^3 (hydrogen) | distribution | liquid density | median | 6.55 g/cm^3 | highest | 17.31 g/cm^3 (gold) | lowest | 1.69 g/cm^3 (beryllium) | distribution | molar volume | median | 16.39 cm^3/mol | highest | 22420 cm^3/mol (helium) | lowest | 4.394 cm^3/mol (boron) | distribution | Mohs hardness | median | 2.25 | highest | 9.3 (between corundum and diamond) (boron) | lowest | 0.5 (between talc and gypsum) (carbon) | distribution | Vickers hardness | all | 216 MPa | 251 MPa | 369 MPa | 1510 MPa | 1670 MPa | 8010 MPa | 9630 MPa | 49000 MPa Brinell hardness | all | 8.83 MPa | 24.5 MPa | 26.4 MPa | 38.3 MPa | 51 MPa | 60 MPa | 94.2 MPa | 180 MPa | 203 MPa | 294 MPa | 412 MPa | 600 MPa | 736 MPa | 874 MPa | 1440 MPa | 2450 MPa | 7270 MPa bulk modulus | median | 42 GPa | highest | 320 GPa (boron) | lowest | 1.1 GPa (chlorine) | distribution | shear modulus | median | 19 GPa | highest | 132 GPa (beryllium) | lowest | 2.8 GPa (thallium) | distribution | Young's modulus | median | 49 GPa | highest | 287 GPa (beryllium) | lowest | 8 GPa (arsenic and thallium) | distribution | Poisson ratio | all | 0.032 | 0.25 | 0.3 | 0.33 | 0.34 | 0.36 | 0.37 | 0.44 | 0.45 ultimate tensile strength | all | 2 MPa | 4 MPa | 7 MPa | 11 MPa | 18 MPa | 37 MPa | 75 MPa | 120 MPa | 140 MPa | 220 MPa | 370 MPa refractive index | median | 1.000895 | highest | 2.417 (carbon) | lowest | 1.000035 (helium) sound speed | median | 2000 m/s | highest | 18350 m/s (carbon) | lowest | 206 m/s (chlorine) | distribution | thermal expansion | median | 1.77×10^-5 K^(-1) | highest | 1.81×10^-4 K^(-1) (mercury) | lowest | 2.6×10^-6 K^(-1) (silicon) | distribution | thermal conductivity | median | 24 W/(m K) | highest | 430 W/(m K) (silver) | lowest | 0.00565 W/(m K) (xenon) | distribution | (properties at standard conditions)