Input interpretation

top 10 chemicals | by short-term exposure limit
Result
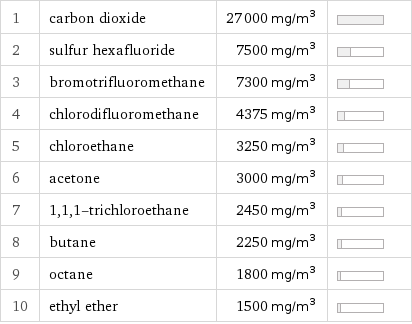
1 | carbon dioxide | 27000 mg/m^3 | 2 | sulfur hexafluoride | 7500 mg/m^3 | 3 | bromotrifluoromethane | 7300 mg/m^3 | 4 | chlorodifluoromethane | 4375 mg/m^3 | 5 | chloroethane | 3250 mg/m^3 | 6 | acetone | 3000 mg/m^3 | 7 | 1, 1, 1-trichloroethane | 2450 mg/m^3 | 8 | butane | 2250 mg/m^3 | 9 | octane | 1800 mg/m^3 | 10 | ethyl ether | 1500 mg/m^3 |
Structure diagrams
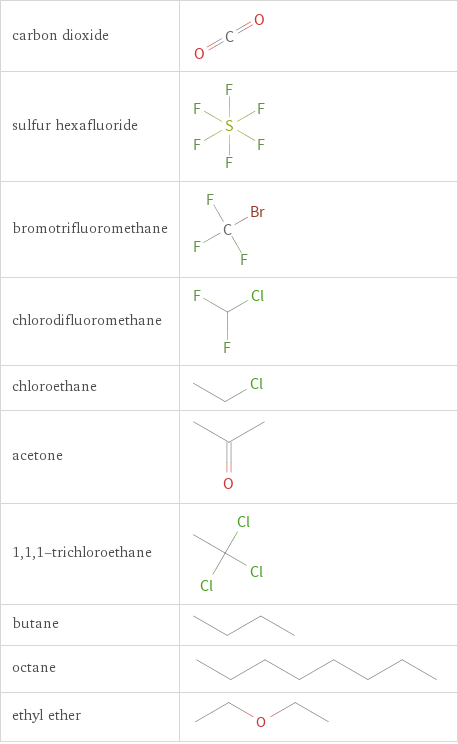
Structure diagrams
Basic properties
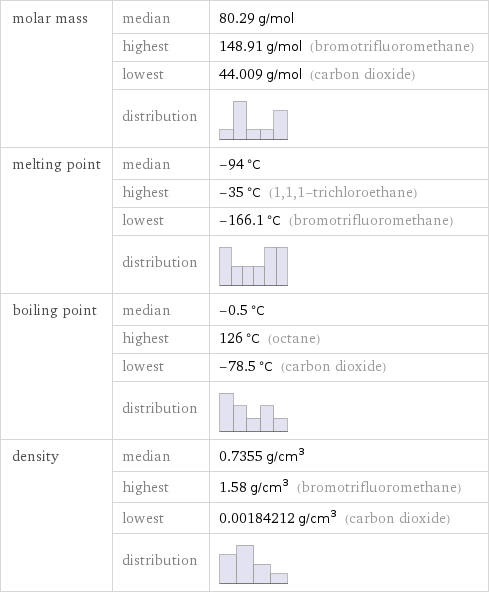
molar mass | median | 80.29 g/mol | highest | 148.91 g/mol (bromotrifluoromethane) | lowest | 44.009 g/mol (carbon dioxide) | distribution | melting point | median | -94 °C | highest | -35 °C (1, 1, 1-trichloroethane) | lowest | -166.1 °C (bromotrifluoromethane) | distribution | boiling point | median | -0.5 °C | highest | 126 °C (octane) | lowest | -78.5 °C (carbon dioxide) | distribution | density | median | 0.7355 g/cm^3 | highest | 1.58 g/cm^3 (bromotrifluoromethane) | lowest | 0.00184212 g/cm^3 (carbon dioxide) | distribution |
Units
