Input interpretation
sodium bromide | nickel(II) nitrate
Chemical names and formulas
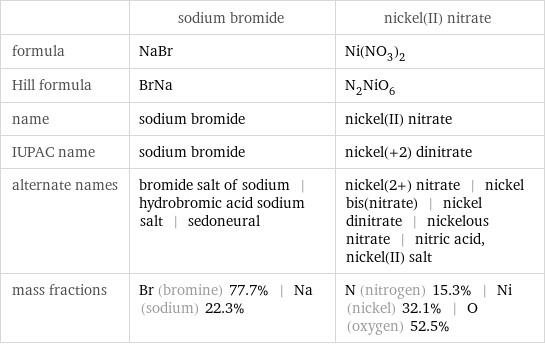
| sodium bromide | nickel(II) nitrate formula | NaBr | Ni(NO_3)_2 Hill formula | BrNa | N_2NiO_6 name | sodium bromide | nickel(II) nitrate IUPAC name | sodium bromide | nickel(+2) dinitrate alternate names | bromide salt of sodium | hydrobromic acid sodium salt | sedoneural | nickel(2+) nitrate | nickel bis(nitrate) | nickel dinitrate | nickelous nitrate | nitric acid, nickel(II) salt mass fractions | Br (bromine) 77.7% | Na (sodium) 22.3% | N (nitrogen) 15.3% | Ni (nickel) 32.1% | O (oxygen) 52.5%
Structure diagrams
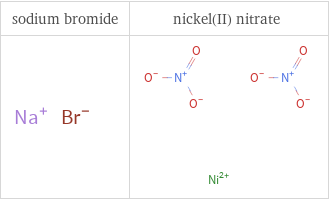
Structure diagrams
Basic properties
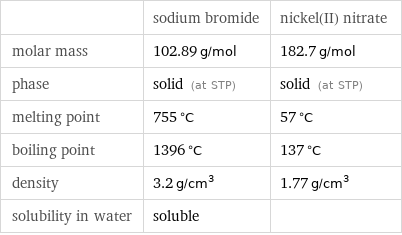
| sodium bromide | nickel(II) nitrate molar mass | 102.89 g/mol | 182.7 g/mol phase | solid (at STP) | solid (at STP) melting point | 755 °C | 57 °C boiling point | 1396 °C | 137 °C density | 3.2 g/cm^3 | 1.77 g/cm^3 solubility in water | soluble |
Units

Thermodynamic properties
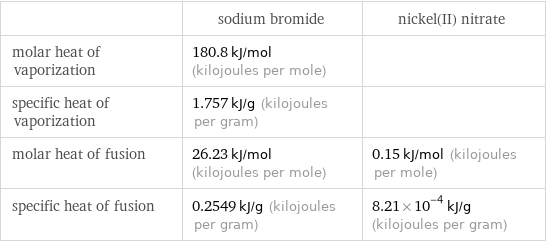
| sodium bromide | nickel(II) nitrate molar heat of vaporization | 180.8 kJ/mol (kilojoules per mole) | specific heat of vaporization | 1.757 kJ/g (kilojoules per gram) | molar heat of fusion | 26.23 kJ/mol (kilojoules per mole) | 0.15 kJ/mol (kilojoules per mole) specific heat of fusion | 0.2549 kJ/g (kilojoules per gram) | 8.21×10^-4 kJ/g (kilojoules per gram)
Solid properties (at STP)
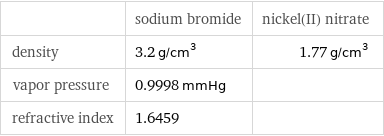
| sodium bromide | nickel(II) nitrate density | 3.2 g/cm^3 | 1.77 g/cm^3 vapor pressure | 0.9998 mmHg | refractive index | 1.6459 |
Units

Chemical identifiers
![| sodium bromide | nickel(II) nitrate CAS number | 7647-15-6 | 13138-45-9 PubChem CID number | 253881 | 25736 PubChem SID number | 24853756 | SMILES identifier | [Na+].[Br-] | [N+](=O)([O-])[O-].[N+](=O)([O-])[O-].[Ni+2]](../image_source/d02866297215c3cc87dd95a249ce8a12.png)
| sodium bromide | nickel(II) nitrate CAS number | 7647-15-6 | 13138-45-9 PubChem CID number | 253881 | 25736 PubChem SID number | 24853756 | SMILES identifier | [Na+].[Br-] | [N+](=O)([O-])[O-].[N+](=O)([O-])[O-].[Ni+2]