Input interpretation

period 2 elements | molar volume
Summary
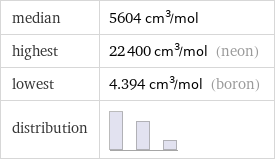
median | 5604 cm^3/mol highest | 22400 cm^3/mol (neon) lowest | 4.394 cm^3/mol (boron) distribution |
Units

Ranked values
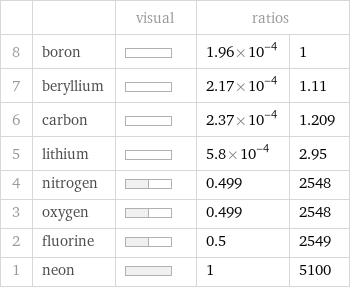
| | visual | ratios | 8 | boron | | 1.96×10^-4 | 1 7 | beryllium | | 2.17×10^-4 | 1.11 6 | carbon | | 2.37×10^-4 | 1.209 5 | lithium | | 5.8×10^-4 | 2.95 4 | nitrogen | | 0.499 | 2548 3 | oxygen | | 0.499 | 2548 2 | fluorine | | 0.5 | 2549 1 | neon | | 1 | 5100
Molar volume rankings
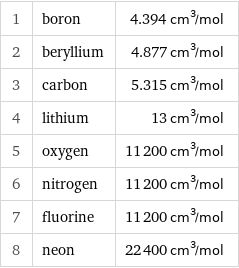
1 | boron | 4.394 cm^3/mol 2 | beryllium | 4.877 cm^3/mol 3 | carbon | 5.315 cm^3/mol 4 | lithium | 13 cm^3/mol 5 | oxygen | 11200 cm^3/mol 6 | nitrogen | 11200 cm^3/mol 7 | fluorine | 11200 cm^3/mol 8 | neon | 22400 cm^3/mol
Unit conversions for median molar volume 5604 cm^3/mol

5.604 M^(-1) (inverse molar)
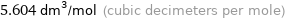
5.604 dm^3/mol (cubic decimeters per mole)
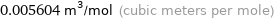
0.005604 m^3/mol (cubic meters per mole)

5.604 L/mol (liters per mole)
Comparison for median molar volume 5604 cm^3/mol
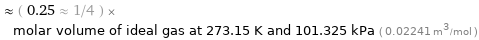
≈ ( 0.25 ≈ 1/4 ) × molar volume of ideal gas at 273.15 K and 101.325 kPa ( 0.02241 m^3/mol )