Input interpretation

top 10 chemicals | by energy content
Result
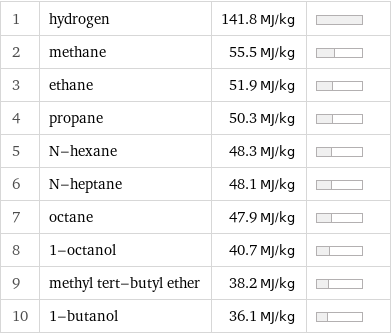
1 | hydrogen | 141.8 MJ/kg | 2 | methane | 55.5 MJ/kg | 3 | ethane | 51.9 MJ/kg | 4 | propane | 50.3 MJ/kg | 5 | N-hexane | 48.3 MJ/kg | 6 | N-heptane | 48.1 MJ/kg | 7 | octane | 47.9 MJ/kg | 8 | 1-octanol | 40.7 MJ/kg | 9 | methyl tert-butyl ether | 38.2 MJ/kg | 10 | 1-butanol | 36.1 MJ/kg |
Structure diagrams
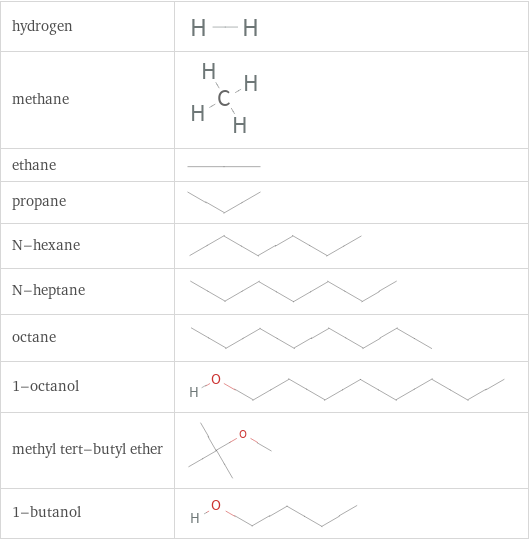
Structure diagrams
Basic properties
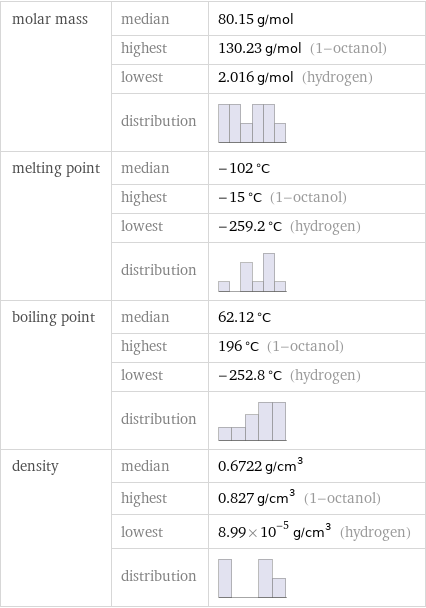
molar mass | median | 80.15 g/mol | highest | 130.23 g/mol (1-octanol) | lowest | 2.016 g/mol (hydrogen) | distribution | melting point | median | -102 °C | highest | -15 °C (1-octanol) | lowest | -259.2 °C (hydrogen) | distribution | boiling point | median | 62.12 °C | highest | 196 °C (1-octanol) | lowest | -252.8 °C (hydrogen) | distribution | density | median | 0.6722 g/cm^3 | highest | 0.827 g/cm^3 (1-octanol) | lowest | 8.99×10^-5 g/cm^3 (hydrogen) | distribution |
Units
