Input interpretation

FeSO_4 (duretter) + K_3Fe(CN)_6 (potassium hexacyanoferrate(III)) ⟶ K_2SO_4 (potassium sulfate) + Fe3(Fe(CN)6)2
Balanced equation

Balance the chemical equation algebraically: FeSO_4 + K_3Fe(CN)_6 ⟶ K_2SO_4 + Fe3(Fe(CN)6)2 Add stoichiometric coefficients, c_i, to the reactants and products: c_1 FeSO_4 + c_2 K_3Fe(CN)_6 ⟶ c_3 K_2SO_4 + c_4 Fe3(Fe(CN)6)2 Set the number of atoms in the reactants equal to the number of atoms in the products for Fe, O, S, C, K and N: Fe: | c_1 + c_2 = 5 c_4 O: | 4 c_1 = 4 c_3 S: | c_1 = c_3 C: | 6 c_2 = 12 c_4 K: | 3 c_2 = 2 c_3 N: | 6 c_2 = 12 c_4 Since the coefficients are relative quantities and underdetermined, choose a coefficient to set arbitrarily. To keep the coefficients small, the arbitrary value is ordinarily one. For instance, set c_4 = 1 and solve the system of equations for the remaining coefficients: c_1 = 3 c_2 = 2 c_3 = 3 c_4 = 1 Substitute the coefficients into the chemical reaction to obtain the balanced equation: Answer: | | 3 FeSO_4 + 2 K_3Fe(CN)_6 ⟶ 3 K_2SO_4 + Fe3(Fe(CN)6)2
Structures
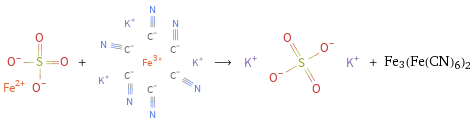
+ ⟶ + Fe3(Fe(CN)6)2
Names
duretter + potassium hexacyanoferrate(III) ⟶ potassium sulfate + Fe3(Fe(CN)6)2
Equilibrium constant
![K_c = ([K2SO4]^3 [Fe3(Fe(CN)6)2])/([FeSO4]^3 [K3Fe(CN)6]^2)](../image_source/12e69cd2e0cccfc7a1078d70eb88291b.png)
K_c = ([K2SO4]^3 [Fe3(Fe(CN)6)2])/([FeSO4]^3 [K3Fe(CN)6]^2)
Rate of reaction
![rate = -1/3 (Δ[FeSO4])/(Δt) = -1/2 (Δ[K3Fe(CN)6])/(Δt) = 1/3 (Δ[K2SO4])/(Δt) = (Δ[Fe3(Fe(CN)6)2])/(Δt) (assuming constant volume and no accumulation of intermediates or side products)](../image_source/0cb17149a480e5bc0d4776cab847dfff.png)
rate = -1/3 (Δ[FeSO4])/(Δt) = -1/2 (Δ[K3Fe(CN)6])/(Δt) = 1/3 (Δ[K2SO4])/(Δt) = (Δ[Fe3(Fe(CN)6)2])/(Δt) (assuming constant volume and no accumulation of intermediates or side products)
Chemical names and formulas
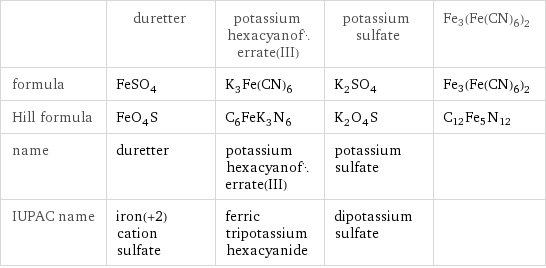
| duretter | potassium hexacyanoferrate(III) | potassium sulfate | Fe3(Fe(CN)6)2 formula | FeSO_4 | K_3Fe(CN)_6 | K_2SO_4 | Fe3(Fe(CN)6)2 Hill formula | FeO_4S | C_6FeK_3N_6 | K_2O_4S | C12Fe5N12 name | duretter | potassium hexacyanoferrate(III) | potassium sulfate | IUPAC name | iron(+2) cation sulfate | ferric tripotassium hexacyanide | dipotassium sulfate |
Substance properties
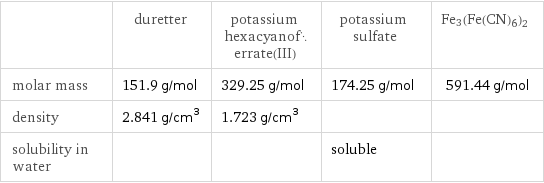
| duretter | potassium hexacyanoferrate(III) | potassium sulfate | Fe3(Fe(CN)6)2 molar mass | 151.9 g/mol | 329.25 g/mol | 174.25 g/mol | 591.44 g/mol density | 2.841 g/cm^3 | 1.723 g/cm^3 | | solubility in water | | | soluble |
Units
