Input interpretation
bromine (chemical element) | iodine (chemical element) | lead (chemical element)
Periodic table location
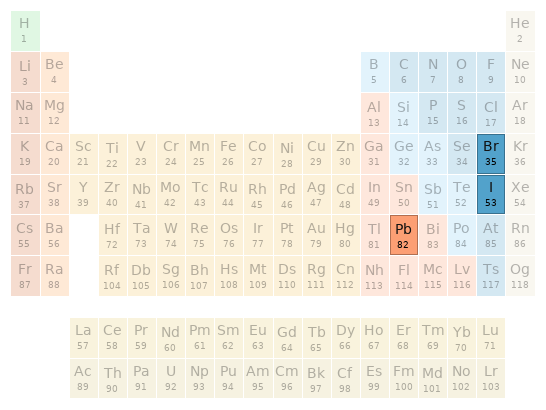
Periodic table location
Images
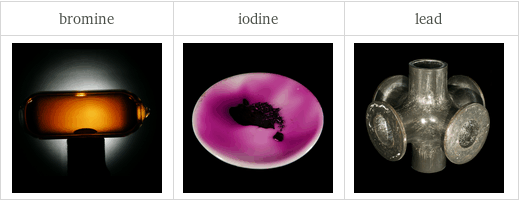
Images
Basic elemental properties
![| bromine | iodine | lead atomic symbol | Br | I | Pb atomic number | 35 | 53 | 82 short electronic configuration | [Ar]4s^23d^104p^5 | [Kr]5s^24d^105p^5 | [Xe]6s^24f^145d^106p^2 Aufbau diagram | 4p 3d 4s | 5p 4d 5s | 6p 5d 4f 6s block | p | p | p group | 17 | 17 | 14 period | 4 | 5 | 6 atomic mass | 79.904 u | 126.90447 u | 207.2 u half-life | (stable) | (stable) | (stable)](../image_source/18f2de0bd1cd6df0dd8ef1efa554526c.png)
| bromine | iodine | lead atomic symbol | Br | I | Pb atomic number | 35 | 53 | 82 short electronic configuration | [Ar]4s^23d^104p^5 | [Kr]5s^24d^105p^5 | [Xe]6s^24f^145d^106p^2 Aufbau diagram | 4p 3d 4s | 5p 4d 5s | 6p 5d 4f 6s block | p | p | p group | 17 | 17 | 14 period | 4 | 5 | 6 atomic mass | 79.904 u | 126.90447 u | 207.2 u half-life | (stable) | (stable) | (stable)
Thermodynamic properties
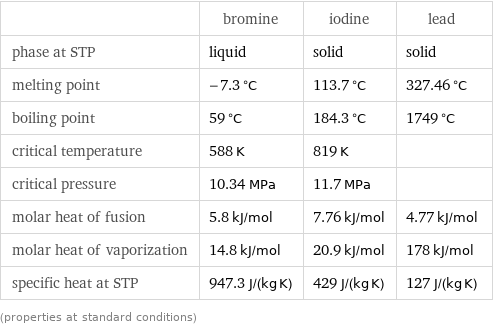
| bromine | iodine | lead phase at STP | liquid | solid | solid melting point | -7.3 °C | 113.7 °C | 327.46 °C boiling point | 59 °C | 184.3 °C | 1749 °C critical temperature | 588 K | 819 K | critical pressure | 10.34 MPa | 11.7 MPa | molar heat of fusion | 5.8 kJ/mol | 7.76 kJ/mol | 4.77 kJ/mol molar heat of vaporization | 14.8 kJ/mol | 20.9 kJ/mol | 178 kJ/mol specific heat at STP | 947.3 J/(kg K) | 429 J/(kg K) | 127 J/(kg K) (properties at standard conditions)
Material properties
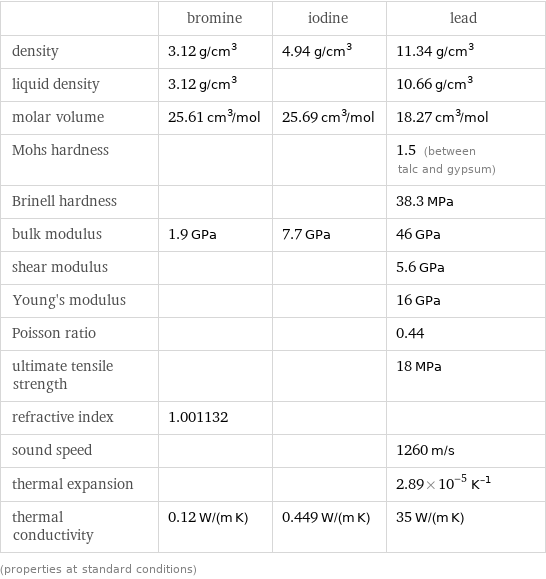
| bromine | iodine | lead density | 3.12 g/cm^3 | 4.94 g/cm^3 | 11.34 g/cm^3 liquid density | 3.12 g/cm^3 | | 10.66 g/cm^3 molar volume | 25.61 cm^3/mol | 25.69 cm^3/mol | 18.27 cm^3/mol Mohs hardness | | | 1.5 (between talc and gypsum) Brinell hardness | | | 38.3 MPa bulk modulus | 1.9 GPa | 7.7 GPa | 46 GPa shear modulus | | | 5.6 GPa Young's modulus | | | 16 GPa Poisson ratio | | | 0.44 ultimate tensile strength | | | 18 MPa refractive index | 1.001132 | | sound speed | | | 1260 m/s thermal expansion | | | 2.89×10^-5 K^(-1) thermal conductivity | 0.12 W/(m K) | 0.449 W/(m K) | 35 W/(m K) (properties at standard conditions)
Electromagnetic properties
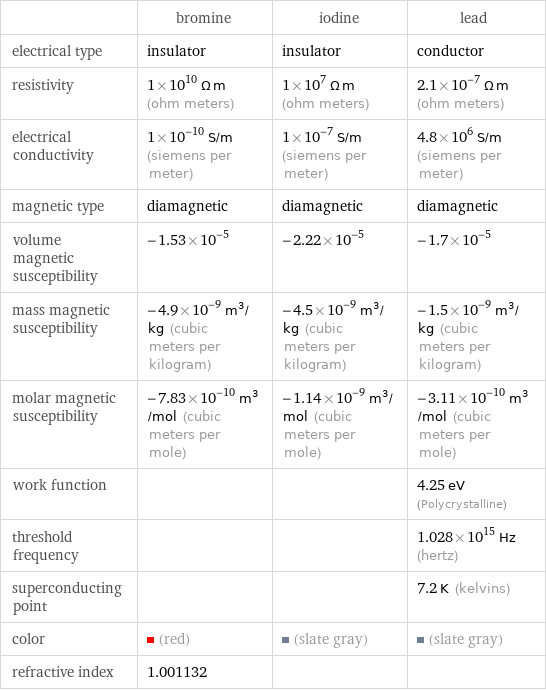
| bromine | iodine | lead electrical type | insulator | insulator | conductor resistivity | 1×10^10 Ω m (ohm meters) | 1×10^7 Ω m (ohm meters) | 2.1×10^-7 Ω m (ohm meters) electrical conductivity | 1×10^-10 S/m (siemens per meter) | 1×10^-7 S/m (siemens per meter) | 4.8×10^6 S/m (siemens per meter) magnetic type | diamagnetic | diamagnetic | diamagnetic volume magnetic susceptibility | -1.53×10^-5 | -2.22×10^-5 | -1.7×10^-5 mass magnetic susceptibility | -4.9×10^-9 m^3/kg (cubic meters per kilogram) | -4.5×10^-9 m^3/kg (cubic meters per kilogram) | -1.5×10^-9 m^3/kg (cubic meters per kilogram) molar magnetic susceptibility | -7.83×10^-10 m^3/mol (cubic meters per mole) | -1.14×10^-9 m^3/mol (cubic meters per mole) | -3.11×10^-10 m^3/mol (cubic meters per mole) work function | | | 4.25 eV (Polycrystalline) threshold frequency | | | 1.028×10^15 Hz (hertz) superconducting point | | | 7.2 K (kelvins) color | (red) | (slate gray) | (slate gray) refractive index | 1.001132 | |
Reactivity
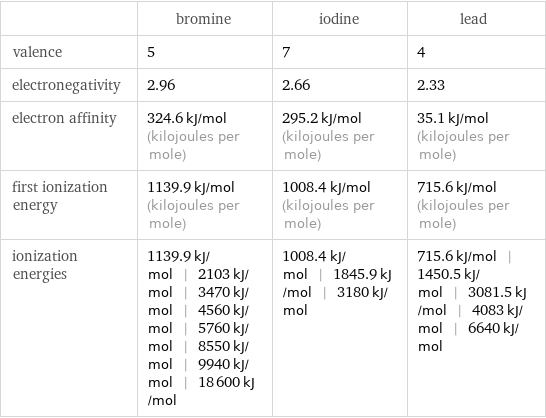
| bromine | iodine | lead valence | 5 | 7 | 4 electronegativity | 2.96 | 2.66 | 2.33 electron affinity | 324.6 kJ/mol (kilojoules per mole) | 295.2 kJ/mol (kilojoules per mole) | 35.1 kJ/mol (kilojoules per mole) first ionization energy | 1139.9 kJ/mol (kilojoules per mole) | 1008.4 kJ/mol (kilojoules per mole) | 715.6 kJ/mol (kilojoules per mole) ionization energies | 1139.9 kJ/mol | 2103 kJ/mol | 3470 kJ/mol | 4560 kJ/mol | 5760 kJ/mol | 8550 kJ/mol | 9940 kJ/mol | 18600 kJ/mol | 1008.4 kJ/mol | 1845.9 kJ/mol | 3180 kJ/mol | 715.6 kJ/mol | 1450.5 kJ/mol | 3081.5 kJ/mol | 4083 kJ/mol | 6640 kJ/mol
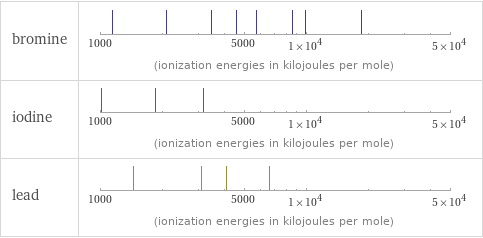
Reactivity
Atomic properties
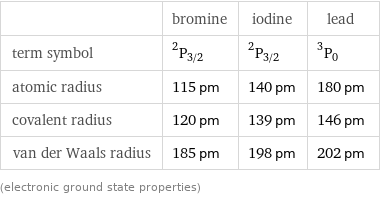
| bromine | iodine | lead term symbol | ^2P_(3/2) | ^2P_(3/2) | ^3P_0 atomic radius | 115 pm | 140 pm | 180 pm covalent radius | 120 pm | 139 pm | 146 pm van der Waals radius | 185 pm | 198 pm | 202 pm (electronic ground state properties)
Abundances
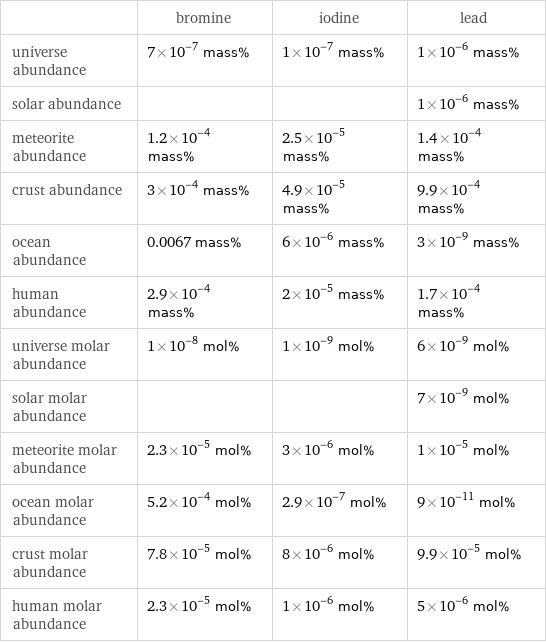
| bromine | iodine | lead universe abundance | 7×10^-7 mass% | 1×10^-7 mass% | 1×10^-6 mass% solar abundance | | | 1×10^-6 mass% meteorite abundance | 1.2×10^-4 mass% | 2.5×10^-5 mass% | 1.4×10^-4 mass% crust abundance | 3×10^-4 mass% | 4.9×10^-5 mass% | 9.9×10^-4 mass% ocean abundance | 0.0067 mass% | 6×10^-6 mass% | 3×10^-9 mass% human abundance | 2.9×10^-4 mass% | 2×10^-5 mass% | 1.7×10^-4 mass% universe molar abundance | 1×10^-8 mol% | 1×10^-9 mol% | 6×10^-9 mol% solar molar abundance | | | 7×10^-9 mol% meteorite molar abundance | 2.3×10^-5 mol% | 3×10^-6 mol% | 1×10^-5 mol% ocean molar abundance | 5.2×10^-4 mol% | 2.9×10^-7 mol% | 9×10^-11 mol% crust molar abundance | 7.8×10^-5 mol% | 8×10^-6 mol% | 9.9×10^-5 mol% human molar abundance | 2.3×10^-5 mol% | 1×10^-6 mol% | 5×10^-6 mol%
Nuclear properties
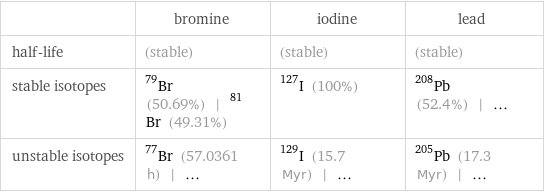
| bromine | iodine | lead half-life | (stable) | (stable) | (stable) stable isotopes | Br-79 (50.69%) | Br-81 (49.31%) | I-127 (100%) | Pb-208 (52.4%) | ... unstable isotopes | Br-77 (57.0361 h) | ... | I-129 (15.7 Myr) | ... | Pb-205 (17.3 Myr) | ...
Units