Input interpretation
hypobromite anion | nitrate anion
Structure diagrams
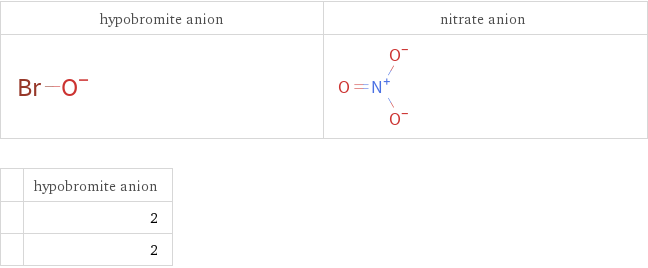
| hypobromite anion | 2 | 2
General properties
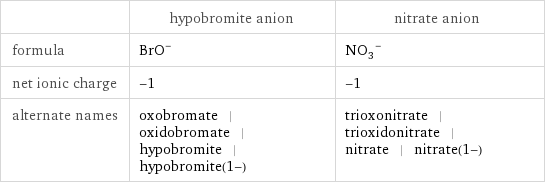
| hypobromite anion | nitrate anion formula | (BrO)^- | (NO_3)^- net ionic charge | -1 | -1 alternate names | oxobromate | oxidobromate | hypobromite | hypobromite(1-) | trioxonitrate | trioxidonitrate | nitrate | nitrate(1-)
Ionic radii

| nitrate anion thermochemical radius | 179 pm
Units

Other properties
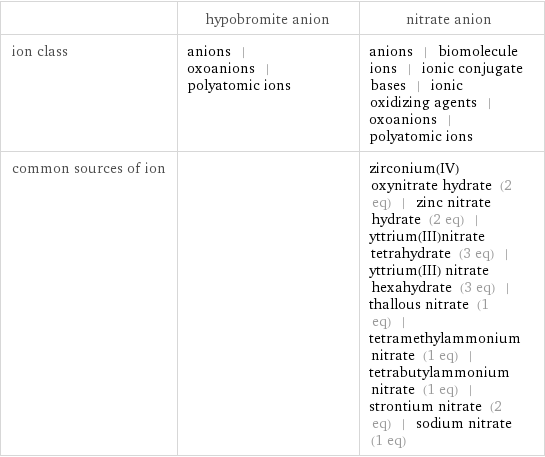
| hypobromite anion | nitrate anion ion class | anions | oxoanions | polyatomic ions | anions | biomolecule ions | ionic conjugate bases | ionic oxidizing agents | oxoanions | polyatomic ions common sources of ion | | zirconium(IV) oxynitrate hydrate (2 eq) | zinc nitrate hydrate (2 eq) | yttrium(III)nitrate tetrahydrate (3 eq) | yttrium(III) nitrate hexahydrate (3 eq) | thallous nitrate (1 eq) | tetramethylammonium nitrate (1 eq) | tetrabutylammonium nitrate (1 eq) | strontium nitrate (2 eq) | sodium nitrate (1 eq)