Input interpretation
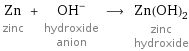
Zn zinc + (OH)^- hydroxide anion ⟶ Zn(OH)_2 zinc hydroxide
Chemical names and formulas
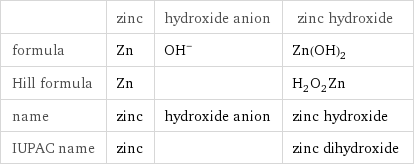
| zinc | hydroxide anion | zinc hydroxide formula | Zn | (OH)^- | Zn(OH)_2 Hill formula | Zn | | H_2O_2Zn name | zinc | hydroxide anion | zinc hydroxide IUPAC name | zinc | | zinc dihydroxide
Substance properties
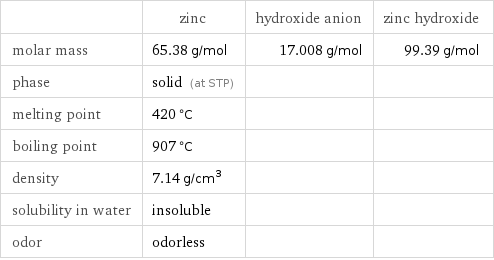
| zinc | hydroxide anion | zinc hydroxide molar mass | 65.38 g/mol | 17.008 g/mol | 99.39 g/mol phase | solid (at STP) | | melting point | 420 °C | | boiling point | 907 °C | | density | 7.14 g/cm^3 | | solubility in water | insoluble | | odor | odorless | |
Units
