Input interpretation

liquid elements (at STP) | atomic diameter
Results

bromine | 230 pm (picometers) mercury | 300 pm (picometers)

liquid elements (at STP) | atomic diameter | 265 pm (picometers) (median)
Relative values
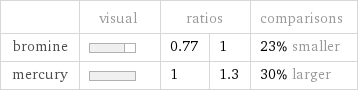
| visual | ratios | | comparisons bromine | | 0.77 | 1 | 23% smaller mercury | | 1 | 1.3 | 30% larger
Plot
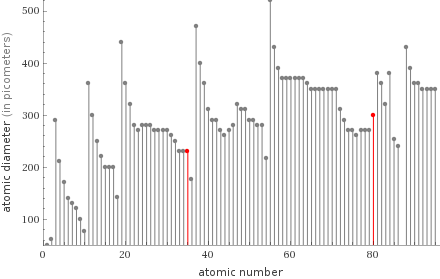
Plot
Periodic table location
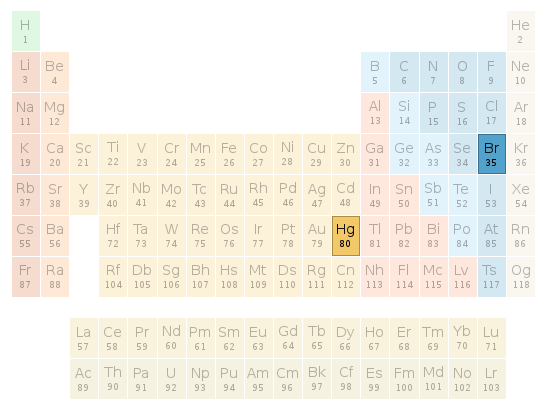
Periodic table location
Atomic radii properties
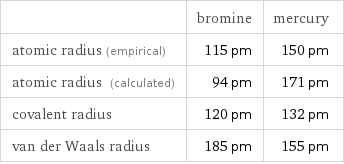
| bromine | mercury atomic radius (empirical) | 115 pm | 150 pm atomic radius (calculated) | 94 pm | 171 pm covalent radius | 120 pm | 132 pm van der Waals radius | 185 pm | 155 pm
Units

Unit conversions for median atomic diameter 265 pm
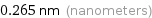
0.265 nm (nanometers)
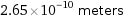
2.65×10^-10 meters
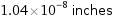
1.04×10^-8 inches
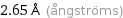
2.65 Å (ångströms)
Comparisons for median atomic diameter 265 pm
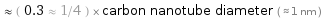
≈ ( 0.3 ≈ 1/4 ) × carbon nanotube diameter ( ≈ 1 nm )
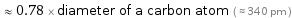
≈ 0.78 × diameter of a carbon atom ( ≈ 340 pm )

≈ (0.00264 to 3.6) × diameter of a molecule ( 0.74 to 1000 Å )