Input interpretation
exploding white dwarf elements
Members
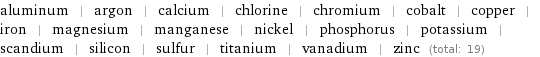
aluminum | argon | calcium | chlorine | chromium | cobalt | copper | iron | magnesium | manganese | nickel | phosphorus | potassium | scandium | silicon | sulfur | titanium | vanadium | zinc (total: 19)
Periodic table location
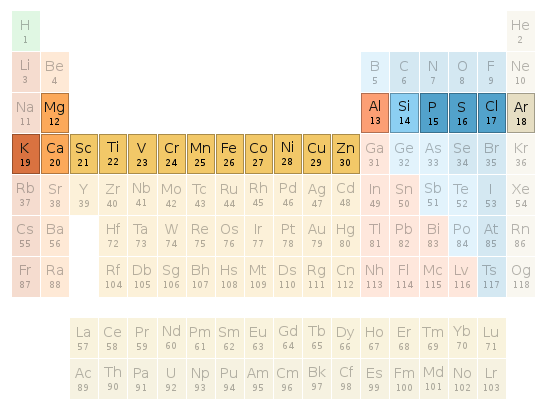
Periodic table location
Images
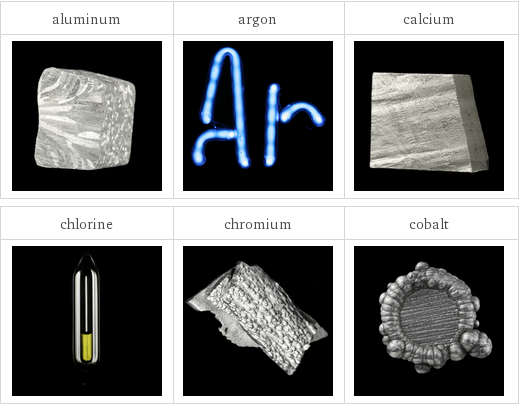
Images
Basic elemental properties
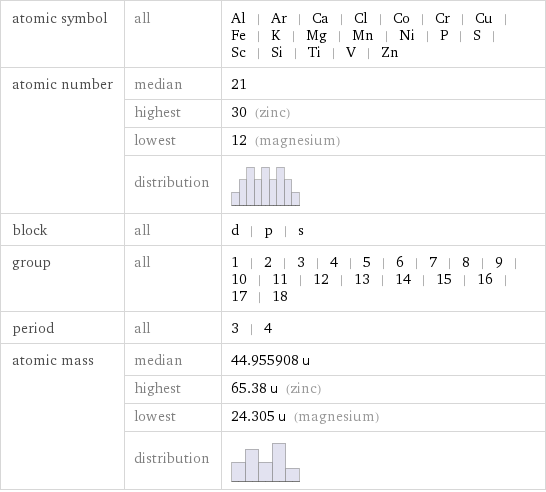
atomic symbol | all | Al | Ar | Ca | Cl | Co | Cr | Cu | Fe | K | Mg | Mn | Ni | P | S | Sc | Si | Ti | V | Zn atomic number | median | 21 | highest | 30 (zinc) | lowest | 12 (magnesium) | distribution | block | all | d | p | s group | all | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 period | all | 3 | 4 atomic mass | median | 44.955908 u | highest | 65.38 u (zinc) | lowest | 24.305 u (magnesium) | distribution |
Thermodynamic properties
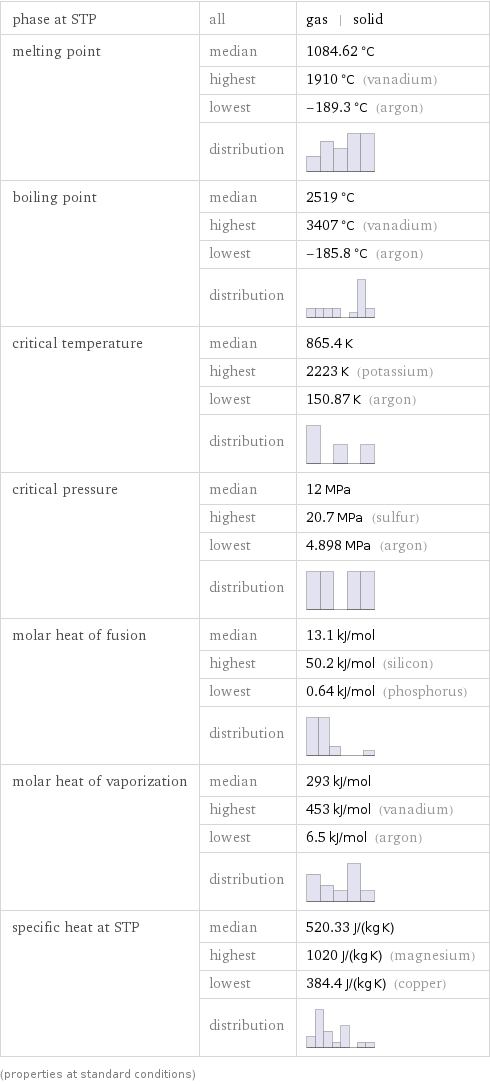
phase at STP | all | gas | solid melting point | median | 1084.62 °C | highest | 1910 °C (vanadium) | lowest | -189.3 °C (argon) | distribution | boiling point | median | 2519 °C | highest | 3407 °C (vanadium) | lowest | -185.8 °C (argon) | distribution | critical temperature | median | 865.4 K | highest | 2223 K (potassium) | lowest | 150.87 K (argon) | distribution | critical pressure | median | 12 MPa | highest | 20.7 MPa (sulfur) | lowest | 4.898 MPa (argon) | distribution | molar heat of fusion | median | 13.1 kJ/mol | highest | 50.2 kJ/mol (silicon) | lowest | 0.64 kJ/mol (phosphorus) | distribution | molar heat of vaporization | median | 293 kJ/mol | highest | 453 kJ/mol (vanadium) | lowest | 6.5 kJ/mol (argon) | distribution | specific heat at STP | median | 520.33 J/(kg K) | highest | 1020 J/(kg K) (magnesium) | lowest | 384.4 J/(kg K) (copper) | distribution | (properties at standard conditions)
Material properties
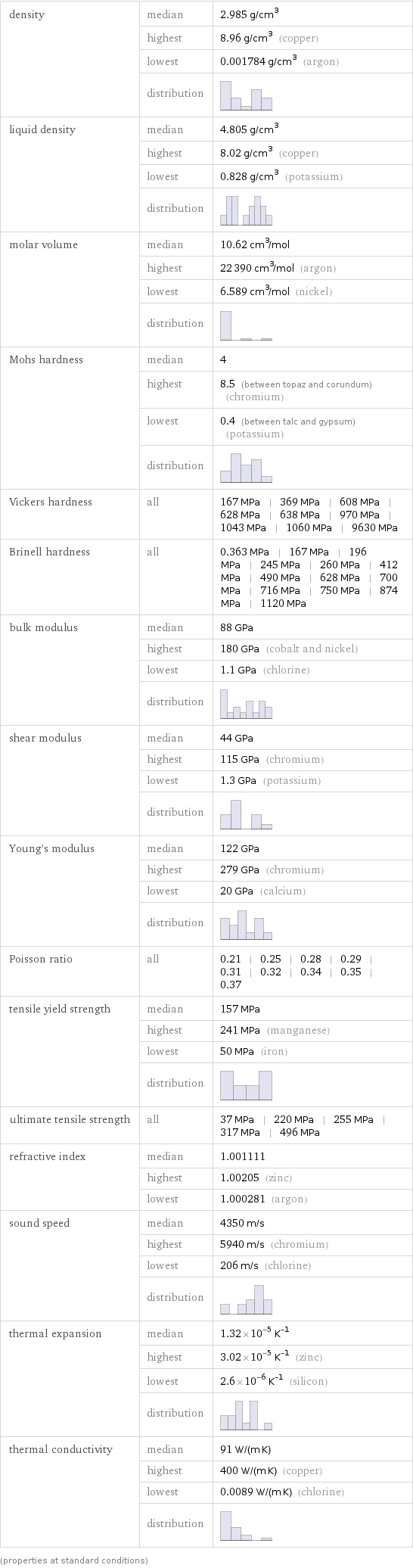
density | median | 2.985 g/cm^3 | highest | 8.96 g/cm^3 (copper) | lowest | 0.001784 g/cm^3 (argon) | distribution | liquid density | median | 4.805 g/cm^3 | highest | 8.02 g/cm^3 (copper) | lowest | 0.828 g/cm^3 (potassium) | distribution | molar volume | median | 10.62 cm^3/mol | highest | 22390 cm^3/mol (argon) | lowest | 6.589 cm^3/mol (nickel) | distribution | Mohs hardness | median | 4 | highest | 8.5 (between topaz and corundum) (chromium) | lowest | 0.4 (between talc and gypsum) (potassium) | distribution | Vickers hardness | all | 167 MPa | 369 MPa | 608 MPa | 628 MPa | 638 MPa | 970 MPa | 1043 MPa | 1060 MPa | 9630 MPa Brinell hardness | all | 0.363 MPa | 167 MPa | 196 MPa | 245 MPa | 260 MPa | 412 MPa | 490 MPa | 628 MPa | 700 MPa | 716 MPa | 750 MPa | 874 MPa | 1120 MPa bulk modulus | median | 88 GPa | highest | 180 GPa (cobalt and nickel) | lowest | 1.1 GPa (chlorine) | distribution | shear modulus | median | 44 GPa | highest | 115 GPa (chromium) | lowest | 1.3 GPa (potassium) | distribution | Young's modulus | median | 122 GPa | highest | 279 GPa (chromium) | lowest | 20 GPa (calcium) | distribution | Poisson ratio | all | 0.21 | 0.25 | 0.28 | 0.29 | 0.31 | 0.32 | 0.34 | 0.35 | 0.37 tensile yield strength | median | 157 MPa | highest | 241 MPa (manganese) | lowest | 50 MPa (iron) | distribution | ultimate tensile strength | all | 37 MPa | 220 MPa | 255 MPa | 317 MPa | 496 MPa refractive index | median | 1.001111 | highest | 1.00205 (zinc) | lowest | 1.000281 (argon) sound speed | median | 4350 m/s | highest | 5940 m/s (chromium) | lowest | 206 m/s (chlorine) | distribution | thermal expansion | median | 1.32×10^-5 K^(-1) | highest | 3.02×10^-5 K^(-1) (zinc) | lowest | 2.6×10^-6 K^(-1) (silicon) | distribution | thermal conductivity | median | 91 W/(m K) | highest | 400 W/(m K) (copper) | lowest | 0.0089 W/(m K) (chlorine) | distribution | (properties at standard conditions)