Input interpretation
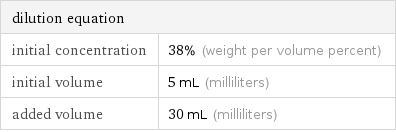
dilution equation | initial concentration | 38% (weight per volume percent) initial volume | 5 mL (milliliters) added volume | 30 mL (milliliters)
Results

final concentration | 1.489 M (molar) = 1.489 mol/dm^3 (moles per cubic decimeter) = 1489 mol/m^3 (moles per cubic meter) = 1.489 mmol/mL (millimoles per milliliter) = 1.489 mol/L (moles per liter) = 148.9 mmol/dL (millimoles per deciliter) = 0.1489 mol/dL (moles per deciliter)
Possible intermediate steps
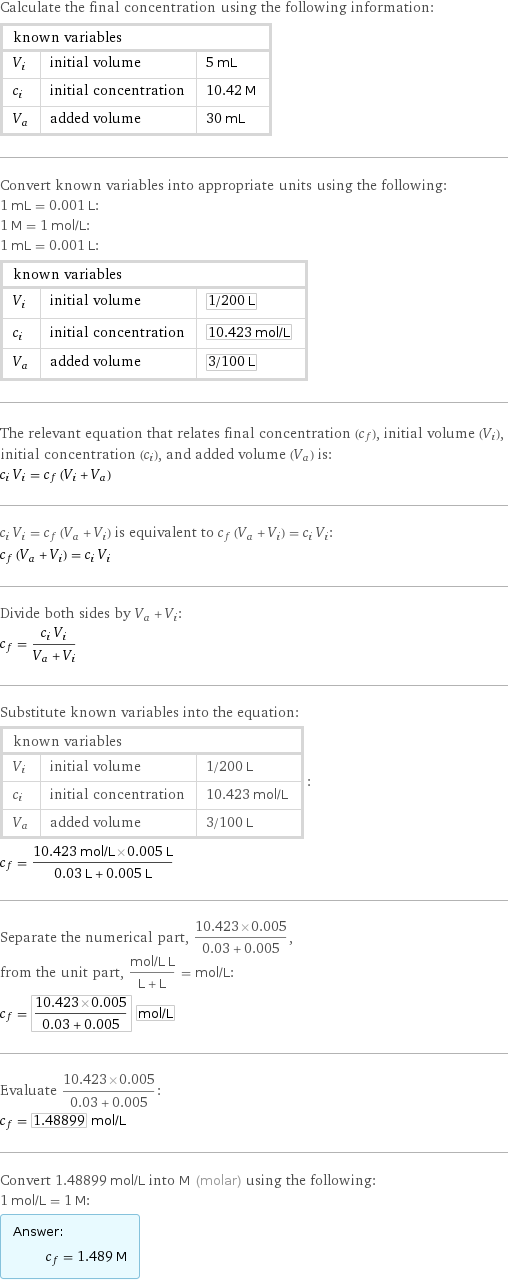
Calculate the final concentration using the following information: known variables | | V_i | initial volume | 5 mL c_i | initial concentration | 10.42 M V_a | added volume | 30 mL Convert known variables into appropriate units using the following: 1 mL = 0.001 L: 1 M = 1 mol/L: 1 mL = 0.001 L: known variables | | V_i | initial volume | 1/200 L c_i | initial concentration | 10.423 mol/L V_a | added volume | 3/100 L The relevant equation that relates final concentration (c_f), initial volume (V_i), initial concentration (c_i), and added volume (V_a) is: c_i V_i = c_f (V_i + V_a) c_i V_i = c_f (V_a + V_i) is equivalent to c_f (V_a + V_i) = c_i V_i: c_f (V_a + V_i) = c_i V_i Divide both sides by V_a + V_i: c_f = (c_i V_i)/(V_a + V_i) Substitute known variables into the equation: known variables | | V_i | initial volume | 1/200 L c_i | initial concentration | 10.423 mol/L V_a | added volume | 3/100 L | : c_f = (10.423 mol/L×0.005 L)/(0.03 L + 0.005 L) Separate the numerical part, (10.423×0.005)/(0.03 + 0.005), from the unit part, (mol/L L)/(L + L) = mol/L: c_f = (10.423×0.005)/(0.03 + 0.005) mol/L Evaluate (10.423×0.005)/(0.03 + 0.005): c_f = 1.48899 mol/L Convert 1.48899 mol/L into M (molar) using the following: 1 mol/L = 1 M: Answer: | | c_f = 1.489 M
Equation
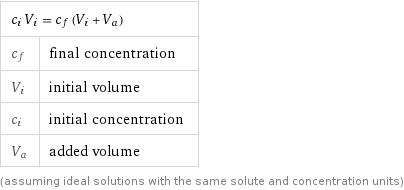
c_i V_i = c_f (V_i + V_a) | c_f | final concentration V_i | initial volume c_i | initial concentration V_a | added volume (assuming ideal solutions with the same solute and concentration units)
Physical properties for 1.489 M hydrogen chloride
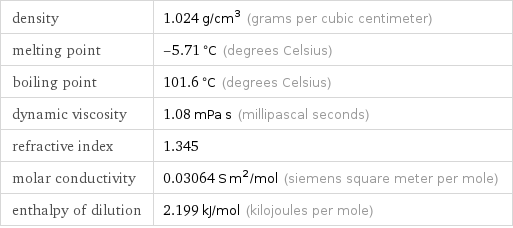
density | 1.024 g/cm^3 (grams per cubic centimeter) melting point | -5.71 °C (degrees Celsius) boiling point | 101.6 °C (degrees Celsius) dynamic viscosity | 1.08 mPa s (millipascal seconds) refractive index | 1.345 molar conductivity | 0.03064 S m^2/mol (siemens square meter per mole) enthalpy of dilution | 2.199 kJ/mol (kilojoules per mole)
Solution properties
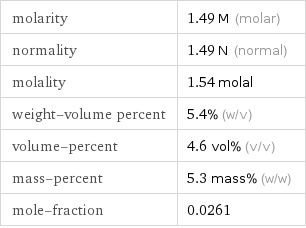
molarity | 1.49 M (molar) normality | 1.49 N (normal) molality | 1.54 molal weight-volume percent | 5.4% (w/v) volume-percent | 4.6 vol% (v/v) mass-percent | 5.3 mass% (w/w) mole-fraction | 0.0261