Input interpretation
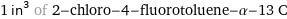
1 in^3 of 2-chloro-4-fluorotoluene-α-13 C
Basic properties for 1 in^3
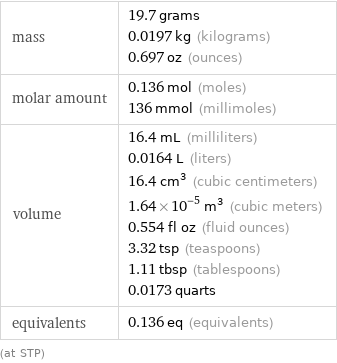
mass | 19.7 grams 0.0197 kg (kilograms) 0.697 oz (ounces) molar amount | 0.136 mol (moles) 136 mmol (millimoles) volume | 16.4 mL (milliliters) 0.0164 L (liters) 16.4 cm^3 (cubic centimeters) 1.64×10^-5 m^3 (cubic meters) 0.554 fl oz (fluid ounces) 3.32 tsp (teaspoons) 1.11 tbsp (tablespoons) 0.0173 quarts equivalents | 0.136 eq (equivalents) (at STP)
Corresponding quantities
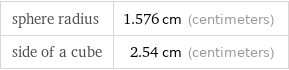
sphere radius | 1.576 cm (centimeters) side of a cube | 2.54 cm (centimeters)
Mass composition for 1 in^3
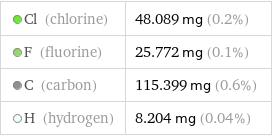
Cl (chlorine) | 48.089 mg (0.2%) F (fluorine) | 25.772 mg (0.1%) C (carbon) | 115.399 mg (0.6%) H (hydrogen) | 8.204 mg (0.04%)
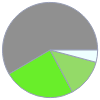
Mass composition for 1 in^3
Lewis structure
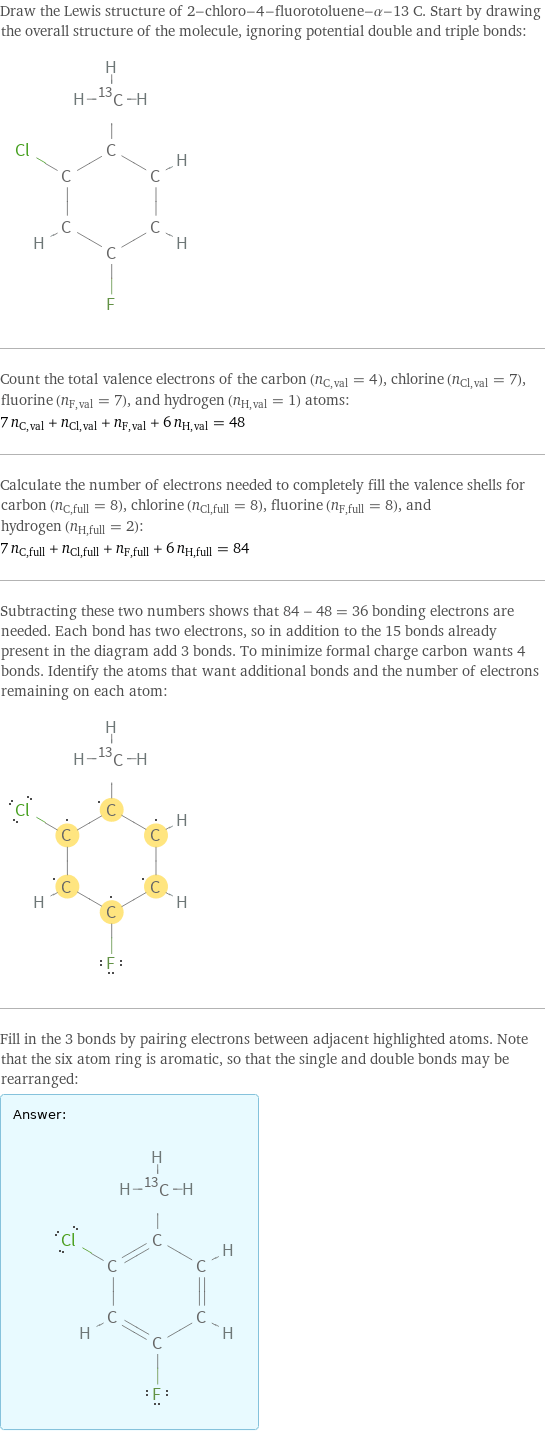
Draw the Lewis structure of 2-chloro-4-fluorotoluene-α-13 C. Start by drawing the overall structure of the molecule, ignoring potential double and triple bonds: Count the total valence electrons of the carbon (n_C, val = 4), chlorine (n_Cl, val = 7), fluorine (n_F, val = 7), and hydrogen (n_H, val = 1) atoms: 7 n_C, val + n_Cl, val + n_F, val + 6 n_H, val = 48 Calculate the number of electrons needed to completely fill the valence shells for carbon (n_C, full = 8), chlorine (n_Cl, full = 8), fluorine (n_F, full = 8), and hydrogen (n_H, full = 2): 7 n_C, full + n_Cl, full + n_F, full + 6 n_H, full = 84 Subtracting these two numbers shows that 84 - 48 = 36 bonding electrons are needed. Each bond has two electrons, so in addition to the 15 bonds already present in the diagram add 3 bonds. To minimize formal charge carbon wants 4 bonds. Identify the atoms that want additional bonds and the number of electrons remaining on each atom: Fill in the 3 bonds by pairing electrons between adjacent highlighted atoms. Note that the six atom ring is aromatic, so that the single and double bonds may be rearranged: Answer: | |
Chemical names and formulas
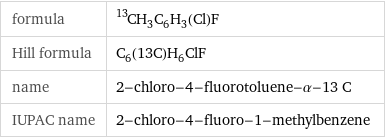
formula | ^13CH_3C_6H_3(Cl)F Hill formula | C_6(13C)H_6ClF name | 2-chloro-4-fluorotoluene-α-13 C IUPAC name | 2-chloro-4-fluoro-1-methylbenzene
Substance properties
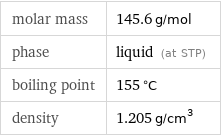
molar mass | 145.6 g/mol phase | liquid (at STP) boiling point | 155 °C density | 1.205 g/cm^3
Units
