Input interpretation
barium hydride
Chemical names and formulas

formula | BaH_2 name | barium hydride IUPAC name | barium(+2) cation; hydrogen(-1) anion alternate names | barium dihydride mass fractions | Ba (barium) 98.6% | H (hydrogen) 1.45%
Structure diagram
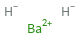
Structure diagram
Basic properties
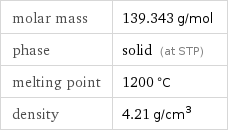
molar mass | 139.343 g/mol phase | solid (at STP) melting point | 1200 °C density | 4.21 g/cm^3
Units

Solid properties (at STP)

density | 4.21 g/cm^3
Units

Thermodynamic properties
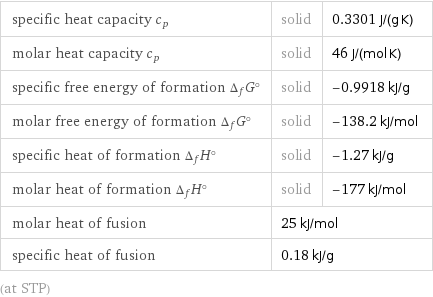
specific heat capacity c_p | solid | 0.3301 J/(g K) molar heat capacity c_p | solid | 46 J/(mol K) specific free energy of formation Δ_fG° | solid | -0.9918 kJ/g molar free energy of formation Δ_fG° | solid | -138.2 kJ/mol specific heat of formation Δ_fH° | solid | -1.27 kJ/g molar heat of formation Δ_fH° | solid | -177 kJ/mol molar heat of fusion | 25 kJ/mol | specific heat of fusion | 0.18 kJ/g | (at STP)
Chemical identifiers
![CAS number | 13477-09-3 PubChem CID number | 83513 SMILES identifier | [H-].[H-].[Ba+2] InChI identifier | InChI=1/Ba.2H/q+2;2*-1 EU number | 236-763-3 Gmelin number | 135955](../image_source/68a377351d1ede3d2d58ae5a724f3576.png)
CAS number | 13477-09-3 PubChem CID number | 83513 SMILES identifier | [H-].[H-].[Ba+2] InChI identifier | InChI=1/Ba.2H/q+2;2*-1 EU number | 236-763-3 Gmelin number | 135955
NFPA label

NFPA label

NFPA hazards | water reactive