Input interpretation
helium (chemical element) | chlorine (chemical element) | argon (chemical element)
Periodic table location
Periodic table location
Images
Images
Basic elemental properties
![| helium | chlorine | argon atomic symbol | He | Cl | Ar atomic number | 2 | 17 | 18 short electronic configuration | 1s^2 | [Ne]3s^23p^5 | [Ne]3s^23p^6 Aufbau diagram | 1s | 3p 3s | 3p 3s block | s | p | p group | 18 | 17 | 18 period | 1 | 3 | 3 atomic mass | 4.002602 u | 35.45 u | 39.948 u half-life | (stable) | (stable) | (stable)](../image_source/da0db94e12fb1ba07c572c7935a2b2c6.png)
| helium | chlorine | argon atomic symbol | He | Cl | Ar atomic number | 2 | 17 | 18 short electronic configuration | 1s^2 | [Ne]3s^23p^5 | [Ne]3s^23p^6 Aufbau diagram | 1s | 3p 3s | 3p 3s block | s | p | p group | 18 | 17 | 18 period | 1 | 3 | 3 atomic mass | 4.002602 u | 35.45 u | 39.948 u half-life | (stable) | (stable) | (stable)
Thermodynamic properties
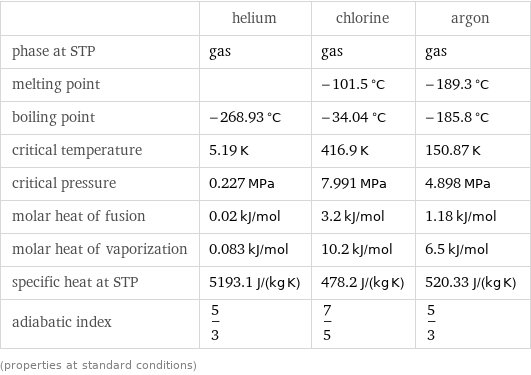
| helium | chlorine | argon phase at STP | gas | gas | gas melting point | | -101.5 °C | -189.3 °C boiling point | -268.93 °C | -34.04 °C | -185.8 °C critical temperature | 5.19 K | 416.9 K | 150.87 K critical pressure | 0.227 MPa | 7.991 MPa | 4.898 MPa molar heat of fusion | 0.02 kJ/mol | 3.2 kJ/mol | 1.18 kJ/mol molar heat of vaporization | 0.083 kJ/mol | 10.2 kJ/mol | 6.5 kJ/mol specific heat at STP | 5193.1 J/(kg K) | 478.2 J/(kg K) | 520.33 J/(kg K) adiabatic index | 5/3 | 7/5 | 5/3 (properties at standard conditions)
Material properties
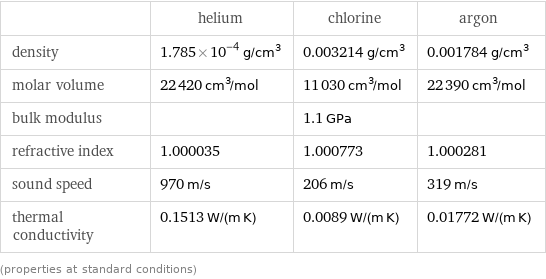
| helium | chlorine | argon density | 1.785×10^-4 g/cm^3 | 0.003214 g/cm^3 | 0.001784 g/cm^3 molar volume | 22420 cm^3/mol | 11030 cm^3/mol | 22390 cm^3/mol bulk modulus | | 1.1 GPa | refractive index | 1.000035 | 1.000773 | 1.000281 sound speed | 970 m/s | 206 m/s | 319 m/s thermal conductivity | 0.1513 W/(m K) | 0.0089 W/(m K) | 0.01772 W/(m K) (properties at standard conditions)
Electromagnetic properties
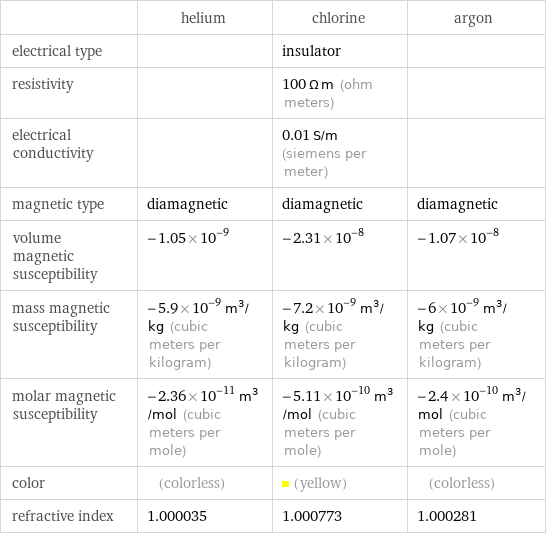
| helium | chlorine | argon electrical type | | insulator | resistivity | | 100 Ω m (ohm meters) | electrical conductivity | | 0.01 S/m (siemens per meter) | magnetic type | diamagnetic | diamagnetic | diamagnetic volume magnetic susceptibility | -1.05×10^-9 | -2.31×10^-8 | -1.07×10^-8 mass magnetic susceptibility | -5.9×10^-9 m^3/kg (cubic meters per kilogram) | -7.2×10^-9 m^3/kg (cubic meters per kilogram) | -6×10^-9 m^3/kg (cubic meters per kilogram) molar magnetic susceptibility | -2.36×10^-11 m^3/mol (cubic meters per mole) | -5.11×10^-10 m^3/mol (cubic meters per mole) | -2.4×10^-10 m^3/mol (cubic meters per mole) color | (colorless) | (yellow) | (colorless) refractive index | 1.000035 | 1.000773 | 1.000281
Reactivity
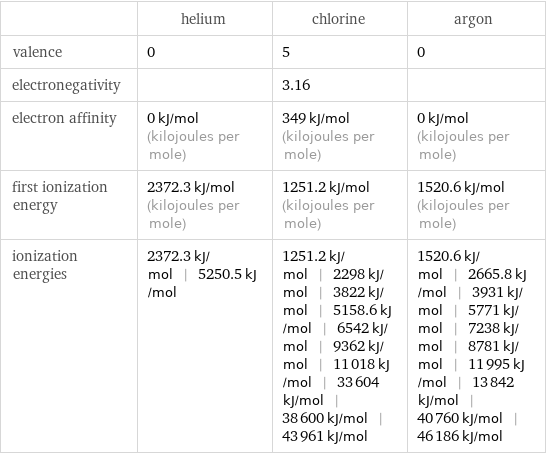
| helium | chlorine | argon valence | 0 | 5 | 0 electronegativity | | 3.16 | electron affinity | 0 kJ/mol (kilojoules per mole) | 349 kJ/mol (kilojoules per mole) | 0 kJ/mol (kilojoules per mole) first ionization energy | 2372.3 kJ/mol (kilojoules per mole) | 1251.2 kJ/mol (kilojoules per mole) | 1520.6 kJ/mol (kilojoules per mole) ionization energies | 2372.3 kJ/mol | 5250.5 kJ/mol | 1251.2 kJ/mol | 2298 kJ/mol | 3822 kJ/mol | 5158.6 kJ/mol | 6542 kJ/mol | 9362 kJ/mol | 11018 kJ/mol | 33604 kJ/mol | 38600 kJ/mol | 43961 kJ/mol | 1520.6 kJ/mol | 2665.8 kJ/mol | 3931 kJ/mol | 5771 kJ/mol | 7238 kJ/mol | 8781 kJ/mol | 11995 kJ/mol | 13842 kJ/mol | 40760 kJ/mol | 46186 kJ/mol
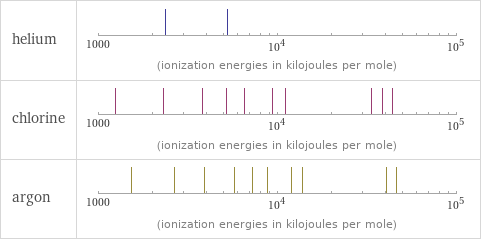
Reactivity
Atomic properties
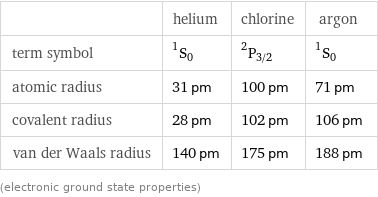
| helium | chlorine | argon term symbol | ^1S_0 | ^2P_(3/2) | ^1S_0 atomic radius | 31 pm | 100 pm | 71 pm covalent radius | 28 pm | 102 pm | 106 pm van der Waals radius | 140 pm | 175 pm | 188 pm (electronic ground state properties)
Abundances
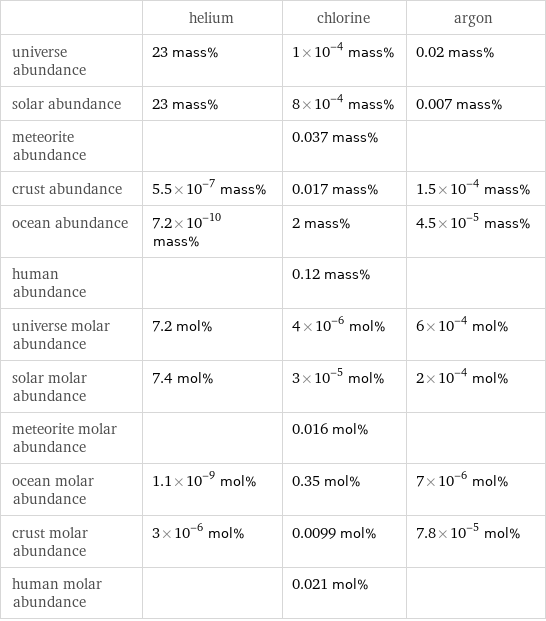
| helium | chlorine | argon universe abundance | 23 mass% | 1×10^-4 mass% | 0.02 mass% solar abundance | 23 mass% | 8×10^-4 mass% | 0.007 mass% meteorite abundance | | 0.037 mass% | crust abundance | 5.5×10^-7 mass% | 0.017 mass% | 1.5×10^-4 mass% ocean abundance | 7.2×10^-10 mass% | 2 mass% | 4.5×10^-5 mass% human abundance | | 0.12 mass% | universe molar abundance | 7.2 mol% | 4×10^-6 mol% | 6×10^-4 mol% solar molar abundance | 7.4 mol% | 3×10^-5 mol% | 2×10^-4 mol% meteorite molar abundance | | 0.016 mol% | ocean molar abundance | 1.1×10^-9 mol% | 0.35 mol% | 7×10^-6 mol% crust molar abundance | 3×10^-6 mol% | 0.0099 mol% | 7.8×10^-5 mol% human molar abundance | | 0.021 mol% |
Nuclear properties
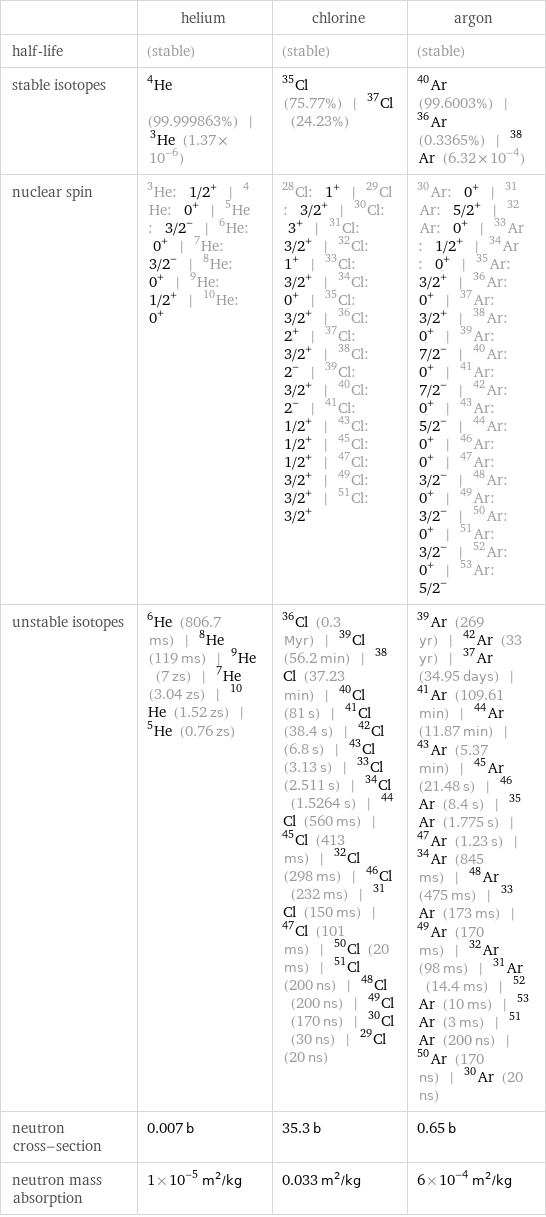
| helium | chlorine | argon half-life | (stable) | (stable) | (stable) stable isotopes | He-4 (99.999863%) | He-3 (1.37×10^-6) | Cl-35 (75.77%) | Cl-37 (24.23%) | Ar-40 (99.6003%) | Ar-36 (0.3365%) | Ar-38 (6.32×10^-4) nuclear spin | He-3: 1/2^+ | He-4: 0^+ | He-5: 3/2^- | He-6: 0^+ | He-7: 3/2^- | He-8: 0^+ | He-9: 1/2^+ | He-10: 0^+ | Cl-28: 1^+ | Cl-29: 3/2^+ | Cl-30: 3^+ | Cl-31: 3/2^+ | Cl-32: 1^+ | Cl-33: 3/2^+ | Cl-34: 0^+ | Cl-35: 3/2^+ | Cl-36: 2^+ | Cl-37: 3/2^+ | Cl-38: 2^- | Cl-39: 3/2^+ | Cl-40: 2^- | Cl-41: 1/2^+ | Cl-43: 1/2^+ | Cl-45: 1/2^+ | Cl-47: 3/2^+ | Cl-49: 3/2^+ | Cl-51: 3/2^+ | Ar-30: 0^+ | Ar-31: 5/2^+ | Ar-32: 0^+ | Ar-33: 1/2^+ | Ar-34: 0^+ | Ar-35: 3/2^+ | Ar-36: 0^+ | Ar-37: 3/2^+ | Ar-38: 0^+ | Ar-39: 7/2^- | Ar-40: 0^+ | Ar-41: 7/2^- | Ar-42: 0^+ | Ar-43: 5/2^- | Ar-44: 0^+ | Ar-46: 0^+ | Ar-47: 3/2^- | Ar-48: 0^+ | Ar-49: 3/2^- | Ar-50: 0^+ | Ar-51: 3/2^- | Ar-52: 0^+ | Ar-53: 5/2^- unstable isotopes | He-6 (806.7 ms) | He-8 (119 ms) | He-9 (7 zs) | He-7 (3.04 zs) | He-10 (1.52 zs) | He-5 (0.76 zs) | Cl-36 (0.3 Myr) | Cl-39 (56.2 min) | Cl-38 (37.23 min) | Cl-40 (81 s) | Cl-41 (38.4 s) | Cl-42 (6.8 s) | Cl-43 (3.13 s) | Cl-33 (2.511 s) | Cl-34 (1.5264 s) | Cl-44 (560 ms) | Cl-45 (413 ms) | Cl-32 (298 ms) | Cl-46 (232 ms) | Cl-31 (150 ms) | Cl-47 (101 ms) | Cl-50 (20 ms) | Cl-51 (200 ns) | Cl-48 (200 ns) | Cl-49 (170 ns) | Cl-30 (30 ns) | Cl-29 (20 ns) | Ar-39 (269 yr) | Ar-42 (33 yr) | Ar-37 (34.95 days) | Ar-41 (109.61 min) | Ar-44 (11.87 min) | Ar-43 (5.37 min) | Ar-45 (21.48 s) | Ar-46 (8.4 s) | Ar-35 (1.775 s) | Ar-47 (1.23 s) | Ar-34 (845 ms) | Ar-48 (475 ms) | Ar-33 (173 ms) | Ar-49 (170 ms) | Ar-32 (98 ms) | Ar-31 (14.4 ms) | Ar-52 (10 ms) | Ar-53 (3 ms) | Ar-51 (200 ns) | Ar-50 (170 ns) | Ar-30 (20 ns) neutron cross-section | 0.007 b | 35.3 b | 0.65 b neutron mass absorption | 1×10^-5 m^2/kg | 0.033 m^2/kg | 6×10^-4 m^2/kg
Identifiers
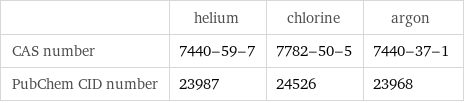
| helium | chlorine | argon CAS number | 7440-59-7 | 7782-50-5 | 7440-37-1 PubChem CID number | 23987 | 24526 | 23968