Input interpretation
Brønsted-Lowry bases | molecular mass
Summary
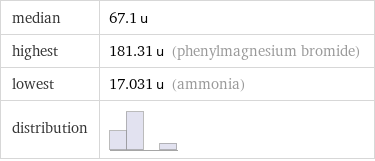
median | 67.1 u highest | 181.31 u (phenylmagnesium bromide) lowest | 17.031 u (ammonia) distribution |
Units

Distribution plots
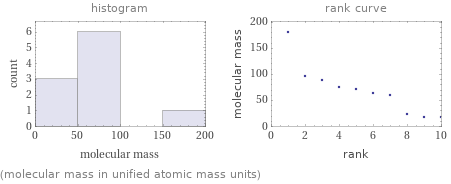
(molecular mass in unified atomic mass units)
Molecular mass rankings
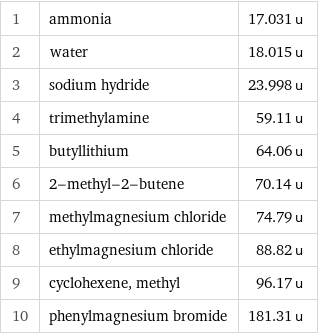
1 | ammonia | 17.031 u 2 | water | 18.015 u 3 | sodium hydride | 23.998 u 4 | trimethylamine | 59.11 u 5 | butyllithium | 64.06 u 6 | 2-methyl-2-butene | 70.14 u 7 | methylmagnesium chloride | 74.79 u 8 | ethylmagnesium chloride | 88.82 u 9 | cyclohexene, methyl | 96.17 u 10 | phenylmagnesium bromide | 181.31 u
Unit conversions for median molecular mass 67.1 u
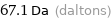
67.1 Da (daltons)
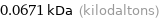
0.0671 kDa (kilodaltons)
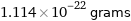
1.114×10^-22 grams
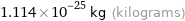
1.114×10^-25 kg (kilograms)
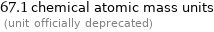
67.1 chemical atomic mass units (unit officially deprecated)
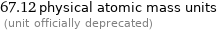
67.12 physical atomic mass units (unit officially deprecated)
Comparisons for median molecular mass 67.1 u

≈ ( 0.093 ≈ 1/11 ) × molecular mass of fullerene ( ≈ 721 u )
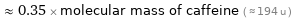
≈ 0.35 × molecular mass of caffeine ( ≈ 194 u )
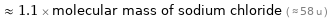
≈ 1.1 × molecular mass of sodium chloride ( ≈ 58 u )
Corresponding quantities
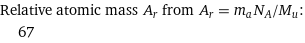
Relative atomic mass A_r from A_r = m_aN_A/M_u: | 67
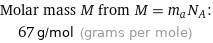
Molar mass M from M = m_aN_A: | 67 g/mol (grams per mole)
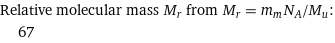
Relative molecular mass M_r from M_r = m_mN_A/M_u: | 67
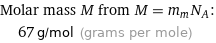
Molar mass M from M = m_mN_A: | 67 g/mol (grams per mole)