Input interpretation
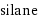
silane
Chemical names and formulas

formula | SiH_4 Hill formula | H_4Si name | silane alternate names | monosilane | silicane | silicon tetrahydride mass fractions | H (hydrogen) 12.6% | Si (silicon) 87.4%
Lewis structure

Draw the Lewis structure of silane. Start by drawing the overall structure of the molecule: Count the total valence electrons of the hydrogen (n_H, val = 1) and silicon (n_Si, val = 4) atoms: 4 n_H, val + n_Si, val = 8 Calculate the number of electrons needed to completely fill the valence shells for hydrogen (n_H, full = 2) and silicon (n_Si, full = 8): 4 n_H, full + n_Si, full = 16 Subtracting these two numbers shows that 16 - 8 = 8 bonding electrons are needed. Each bond has two electrons, so the above diagram has all the necessary bonds. There are 4 bonds and hence 8 bonding electrons in the diagram. Lastly, fill in the remaining unbonded electrons on each atom. In total, there remain 8 - 8 = 0 electrons left to draw and the diagram is complete: Answer: | |
3D structure
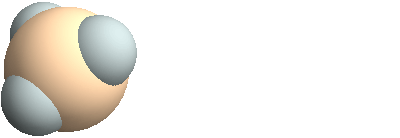
3D structure
Basic properties
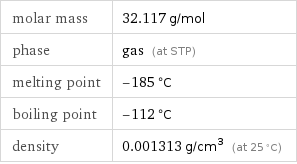
molar mass | 32.117 g/mol phase | gas (at STP) melting point | -185 °C boiling point | -112 °C density | 0.001313 g/cm^3 (at 25 °C)
Units

Gas properties (at STP)
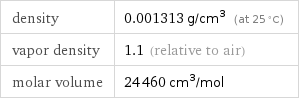
density | 0.001313 g/cm^3 (at 25 °C) vapor density | 1.1 (relative to air) molar volume | 24460 cm^3/mol
Units

Thermodynamic properties

molar heat of fusion | 0.67 kJ/mol specific heat of fusion | 0.02086 kJ/g (at STP)
Chemical identifiers
![CAS number | 7803-62-5 PubChem CID number | 23953 SMILES identifier | [SiH4] RTECS number | VV1400000 MDL number | MFCD00011228](../image_source/69c0923159d6ee6c0a959ecd4f8d2252.png)
CAS number | 7803-62-5 PubChem CID number | 23953 SMILES identifier | [SiH4] RTECS number | VV1400000 MDL number | MFCD00011228
Toxicity properties

short-term exposure limit | 1.5 mg/m^3

long-term exposure limit | 0.7 mg/m^3 (over 8 hours)