Input interpretation
aluminum fluoride | 1, 1, 2, 2-tetrafluoroethane
Chemical names and formulas
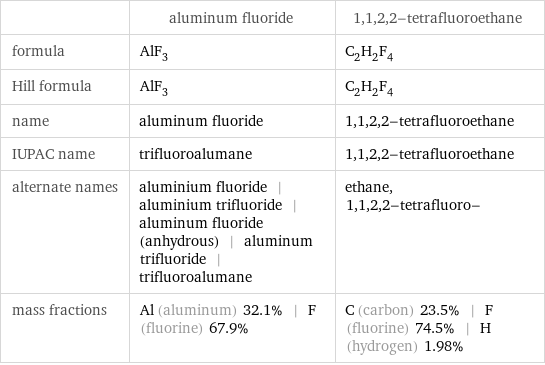
| aluminum fluoride | 1, 1, 2, 2-tetrafluoroethane formula | AlF_3 | C_2H_2F_4 Hill formula | AlF_3 | C_2H_2F_4 name | aluminum fluoride | 1, 1, 2, 2-tetrafluoroethane IUPAC name | trifluoroalumane | 1, 1, 2, 2-tetrafluoroethane alternate names | aluminium fluoride | aluminium trifluoride | aluminum fluoride (anhydrous) | aluminum trifluoride | trifluoroalumane | ethane, 1, 1, 2, 2-tetrafluoro- mass fractions | Al (aluminum) 32.1% | F (fluorine) 67.9% | C (carbon) 23.5% | F (fluorine) 74.5% | H (hydrogen) 1.98%
Structure diagrams
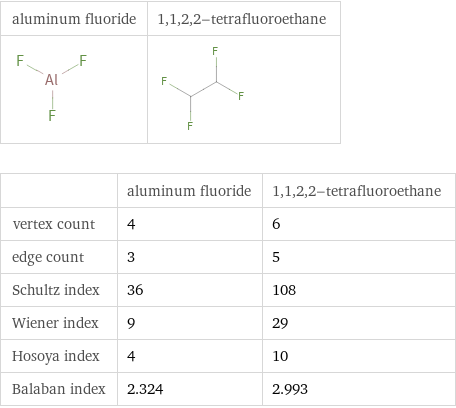
| aluminum fluoride | 1, 1, 2, 2-tetrafluoroethane vertex count | 4 | 6 edge count | 3 | 5 Schultz index | 36 | 108 Wiener index | 9 | 29 Hosoya index | 4 | 10 Balaban index | 2.324 | 2.993
3D structure
3D structure
Basic properties
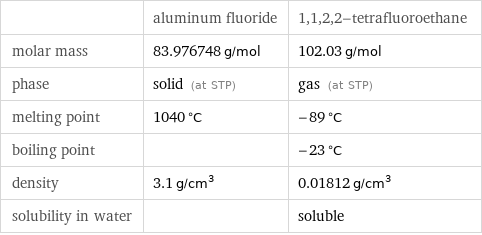
| aluminum fluoride | 1, 1, 2, 2-tetrafluoroethane molar mass | 83.976748 g/mol | 102.03 g/mol phase | solid (at STP) | gas (at STP) melting point | 1040 °C | -89 °C boiling point | | -23 °C density | 3.1 g/cm^3 | 0.01812 g/cm^3 solubility in water | | soluble
Units

Gas properties
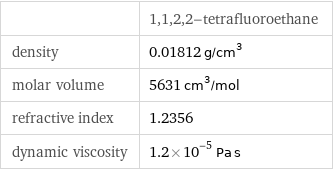
| 1, 1, 2, 2-tetrafluoroethane density | 0.01812 g/cm^3 molar volume | 5631 cm^3/mol refractive index | 1.2356 dynamic viscosity | 1.2×10^-5 Pa s
Units
Thermodynamic properties
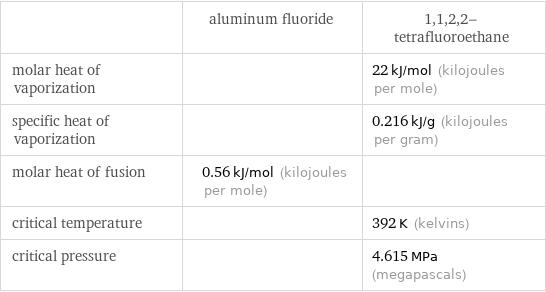
| aluminum fluoride | 1, 1, 2, 2-tetrafluoroethane molar heat of vaporization | | 22 kJ/mol (kilojoules per mole) specific heat of vaporization | | 0.216 kJ/g (kilojoules per gram) molar heat of fusion | 0.56 kJ/mol (kilojoules per mole) | critical temperature | | 392 K (kelvins) critical pressure | | 4.615 MPa (megapascals)
Solid properties
| aluminum fluoride density | 3.1 g/cm^3 vapor pressure | 16.4 mmHg
Units
