Input interpretation
anions | ionic radius
Summary
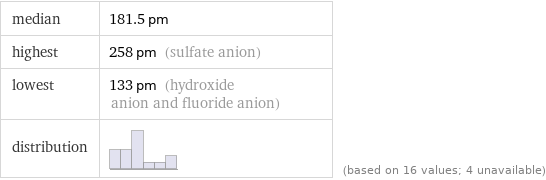
median | 181.5 pm highest | 258 pm (sulfate anion) lowest | 133 pm (hydroxide anion and fluoride anion) distribution | | (based on 16 values; 4 unavailable)
Entities with missing values

hydride anion | chlorite anion | dichromate anion | manganate anion (total: 4)
Distribution plots
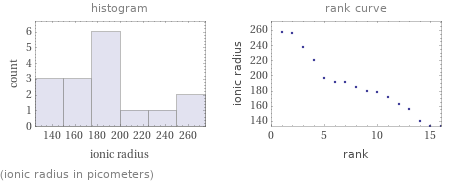
(ionic radius in picometers)
Ionic radius rankings
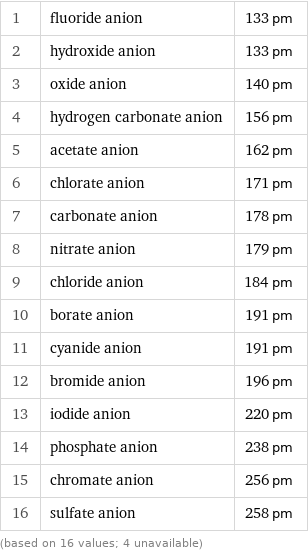
1 | fluoride anion | 133 pm 2 | hydroxide anion | 133 pm 3 | oxide anion | 140 pm 4 | hydrogen carbonate anion | 156 pm 5 | acetate anion | 162 pm 6 | chlorate anion | 171 pm 7 | carbonate anion | 178 pm 8 | nitrate anion | 179 pm 9 | chloride anion | 184 pm 10 | borate anion | 191 pm 11 | cyanide anion | 191 pm 12 | bromide anion | 196 pm 13 | iodide anion | 220 pm 14 | phosphate anion | 238 pm 15 | chromate anion | 256 pm 16 | sulfate anion | 258 pm (based on 16 values; 4 unavailable)
Ionic radii
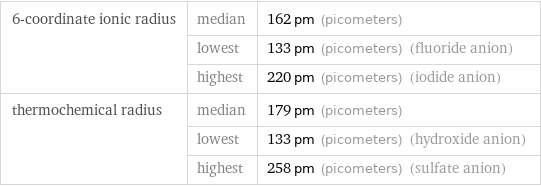
6-coordinate ionic radius | median | 162 pm (picometers) | lowest | 133 pm (picometers) (fluoride anion) | highest | 220 pm (picometers) (iodide anion) thermochemical radius | median | 179 pm (picometers) | lowest | 133 pm (picometers) (hydroxide anion) | highest | 258 pm (picometers) (sulfate anion)
Unit conversions for median ionic radius 181.5 pm
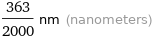
363/2000 nm (nanometers)
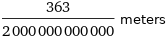
363/2000000000000 meters
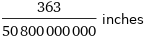
363/50800000000 inches
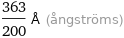
363/200 Å (ångströms)