Input interpretation
ionic conjugate bases
Members

acetate anion | benzoate anion | carbonate anion | dihydrogen phosphate anion | hydrogen carbonate anion | hydrogen phosphate anion | hydrogen sulfate anion | hydrosulfide anion | lactate anion | nitrate anion | nitrilotriacetate anion | nitrite anion | phosphate anion | salicylate anion | stearate anion | succinate anion | sulfate anion | tosylate anion | triflate anion | trifluoroacetate anion (total: 20)
Ionic radii
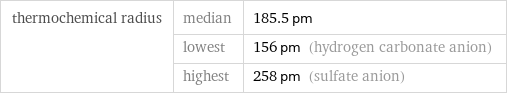
thermochemical radius | median | 185.5 pm | lowest | 156 pm (hydrogen carbonate anion) | highest | 258 pm (sulfate anion)
Units
