Input interpretation
cadmium sulfate
Chemical names and formulas

formula | CdSO_4 Hill formula | CdO_4S name | cadmium sulfate alternate names | cadmium; sulfuric acid; hydrate mass fractions | Cd (cadmium) 53.9% | O (oxygen) 30.7% | S (sulfur) 15.4%
Structure diagram
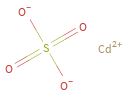
Structure diagram
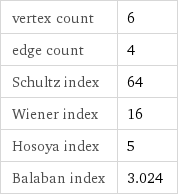
vertex count | 6 edge count | 4 Schultz index | 64 Wiener index | 16 Hosoya index | 5 Balaban index | 3.024
Basic properties
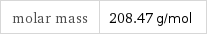
molar mass | 208.47 g/mol
Units

Thermodynamic properties
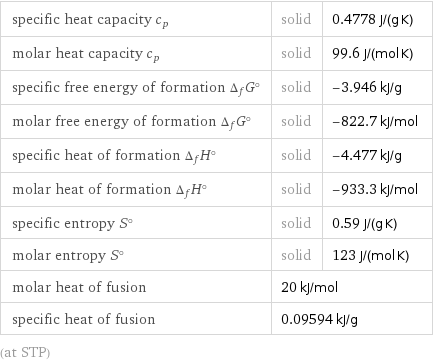
specific heat capacity c_p | solid | 0.4778 J/(g K) molar heat capacity c_p | solid | 99.6 J/(mol K) specific free energy of formation Δ_fG° | solid | -3.946 kJ/g molar free energy of formation Δ_fG° | solid | -822.7 kJ/mol specific heat of formation Δ_fH° | solid | -4.477 kJ/g molar heat of formation Δ_fH° | solid | -933.3 kJ/mol specific entropy S° | solid | 0.59 J/(g K) molar entropy S° | solid | 123 J/(mol K) molar heat of fusion | 20 kJ/mol | specific heat of fusion | 0.09594 kJ/g | (at STP)
Chemical identifiers
![CAS number | 10124-36-4 SMILES identifier | [Cd+2].O=S(=O)([O-])[O-] RTECS number | EV2700000 MDL number | MFCD00010923](../image_source/72c051336ebff6bea3abbbf9e1afae7c.png)
CAS number | 10124-36-4 SMILES identifier | [Cd+2].O=S(=O)([O-])[O-] RTECS number | EV2700000 MDL number | MFCD00010923
Toxicity properties
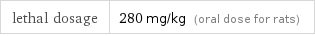
lethal dosage | 280 mg/kg (oral dose for rats)

probable lethal dose for man | 30 mL (milliliters)