Input interpretation
osmium tetroxide
Chemical names and formulas
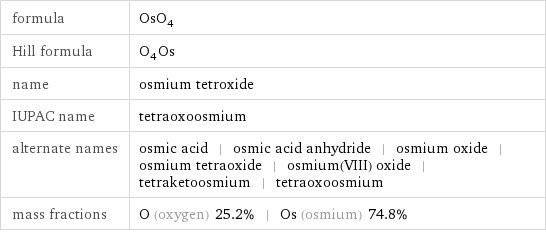
formula | OsO_4 Hill formula | O_4Os name | osmium tetroxide IUPAC name | tetraoxoosmium alternate names | osmic acid | osmic acid anhydride | osmium oxide | osmium tetraoxide | osmium(VIII) oxide | tetraketoosmium | tetraoxoosmium mass fractions | O (oxygen) 25.2% | Os (osmium) 74.8%
Structure diagram
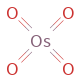
Structure diagram
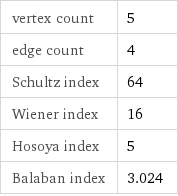
vertex count | 5 edge count | 4 Schultz index | 64 Wiener index | 16 Hosoya index | 5 Balaban index | 3.024
Basic properties
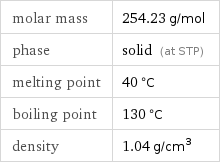
molar mass | 254.23 g/mol phase | solid (at STP) melting point | 40 °C boiling point | 130 °C density | 1.04 g/cm^3
Units

Solid properties (at STP)
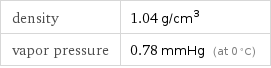
density | 1.04 g/cm^3 vapor pressure | 0.78 mmHg (at 0 °C)
Units

Thermodynamic properties
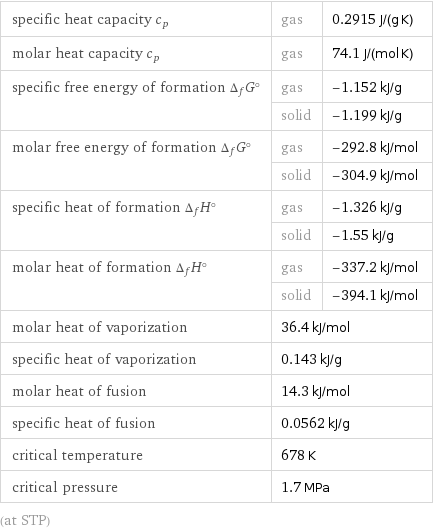
specific heat capacity c_p | gas | 0.2915 J/(g K) molar heat capacity c_p | gas | 74.1 J/(mol K) specific free energy of formation Δ_fG° | gas | -1.152 kJ/g | solid | -1.199 kJ/g molar free energy of formation Δ_fG° | gas | -292.8 kJ/mol | solid | -304.9 kJ/mol specific heat of formation Δ_fH° | gas | -1.326 kJ/g | solid | -1.55 kJ/g molar heat of formation Δ_fH° | gas | -337.2 kJ/mol | solid | -394.1 kJ/mol molar heat of vaporization | 36.4 kJ/mol | specific heat of vaporization | 0.143 kJ/g | molar heat of fusion | 14.3 kJ/mol | specific heat of fusion | 0.0562 kJ/g | critical temperature | 678 K | critical pressure | 1.7 MPa | (at STP)
Chemical identifiers
(=O)=O InChI identifier | InChI=1/4O.Os/rO4Os/c1-5(2, 3)4 MDL number | MFCD00011150](../image_source/7b56db91ebe404a39b77487702eb5575.png)
CAS number | 20816-12-0 PubChem CID number | 30318 PubChem SID number | 24884370 SMILES identifier | O=[Os](=O)(=O)=O InChI identifier | InChI=1/4O.Os/rO4Os/c1-5(2, 3)4 MDL number | MFCD00011150
NFPA label

NFPA label

NFPA health rating | 3 NFPA fire rating | 3 NFPA reactivity rating | 0
Safety properties

flash point | -40 °C
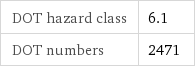
DOT hazard class | 6.1 DOT numbers | 2471
Toxicity properties
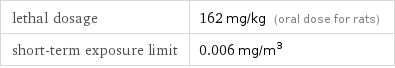
lethal dosage | 162 mg/kg (oral dose for rats) short-term exposure limit | 0.006 mg/m^3

probable lethal dose for man | 30 mL (milliliters) long-term exposure limit | 0.002 mg/m^3 (over 8 hours) RTECS classes | drug | mutagen | reproductive effector | human data