Input interpretation
rare earth metals | number of valence electrons
Summary

median | 2 e highest | 2 e (17 elements) lowest | 2 e (17 elements)
Units

Number of valence electrons rankings
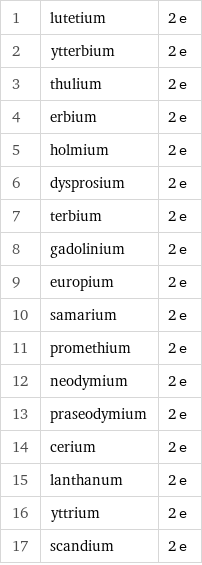
1 | lutetium | 2 e 2 | ytterbium | 2 e 3 | thulium | 2 e 4 | erbium | 2 e 5 | holmium | 2 e 6 | dysprosium | 2 e 7 | terbium | 2 e 8 | gadolinium | 2 e 9 | europium | 2 e 10 | samarium | 2 e 11 | promethium | 2 e 12 | neodymium | 2 e 13 | praseodymium | 2 e 14 | cerium | 2 e 15 | lanthanum | 2 e 16 | yttrium | 2 e 17 | scandium | 2 e
Corresponding quantity
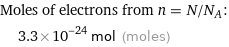
Moles of electrons from n = N/N_A: | 3.3×10^-24 mol (moles)