Input interpretation

alkyl halides | molar heat of fusion
Summary
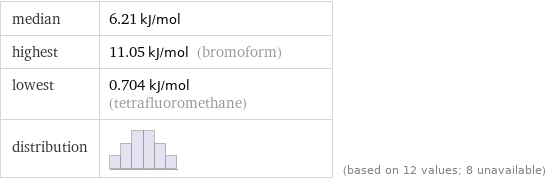
median | 6.21 kJ/mol highest | 11.05 kJ/mol (bromoform) lowest | 0.704 kJ/mol (tetrafluoromethane) distribution | | (based on 12 values; 8 unavailable)
Entities with missing values

fluoromethane | ethyl fluoride | difluoroethane | sec-butyl chloride | 1, 1, 2, 2-tetrafluoroethane | 1-fluorohexane | 1, 1-dibromoethane | 1, 1, 2, 2-tetrabromoethane (total: 8)
Distribution plots
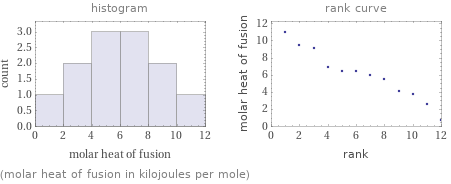
(molar heat of fusion in kilojoules per mole)
Molar heat of fusion rankings
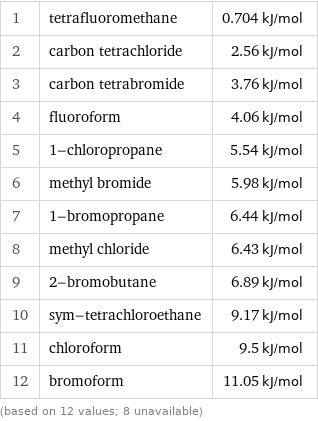
1 | tetrafluoromethane | 0.704 kJ/mol 2 | carbon tetrachloride | 2.56 kJ/mol 3 | carbon tetrabromide | 3.76 kJ/mol 4 | fluoroform | 4.06 kJ/mol 5 | 1-chloropropane | 5.54 kJ/mol 6 | methyl bromide | 5.98 kJ/mol 7 | 1-bromopropane | 6.44 kJ/mol 8 | methyl chloride | 6.43 kJ/mol 9 | 2-bromobutane | 6.89 kJ/mol 10 | sym-tetrachloroethane | 9.17 kJ/mol 11 | chloroform | 9.5 kJ/mol 12 | bromoform | 11.05 kJ/mol (based on 12 values; 8 unavailable)
Unit conversions for median molar heat of fusion 6.21 kJ/mol
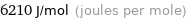
6210 J/mol (joules per mole)

1.48 kcal/mol (thermochemical kilocalories per mole)
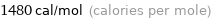
1480 cal/mol (calories per mole)

0.0644 eV (molar electronvolts)