Input interpretation
barium oxide | sodium formate
Chemical names and formulas
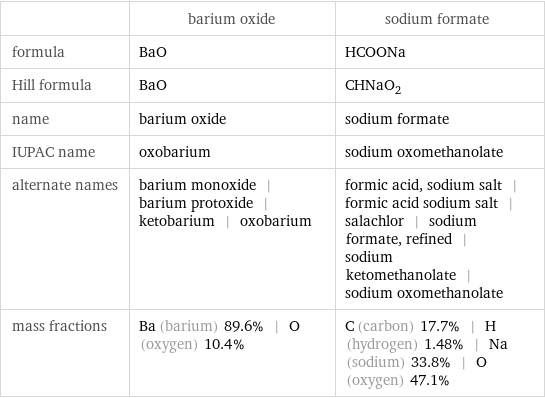
| barium oxide | sodium formate formula | BaO | HCOONa Hill formula | BaO | CHNaO_2 name | barium oxide | sodium formate IUPAC name | oxobarium | sodium oxomethanolate alternate names | barium monoxide | barium protoxide | ketobarium | oxobarium | formic acid, sodium salt | formic acid sodium salt | salachlor | sodium formate, refined | sodium ketomethanolate | sodium oxomethanolate mass fractions | Ba (barium) 89.6% | O (oxygen) 10.4% | C (carbon) 17.7% | H (hydrogen) 1.48% | Na (sodium) 33.8% | O (oxygen) 47.1%
Structure diagrams
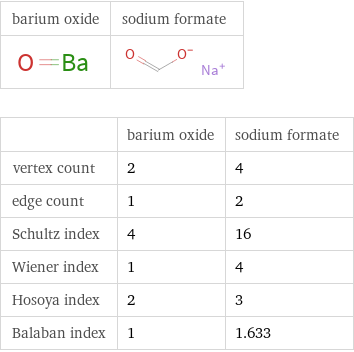
| barium oxide | sodium formate vertex count | 2 | 4 edge count | 1 | 2 Schultz index | 4 | 16 Wiener index | 1 | 4 Hosoya index | 2 | 3 Balaban index | 1 | 1.633
Basic properties
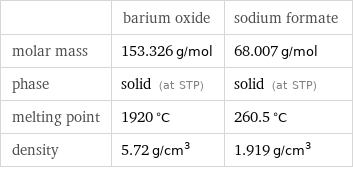
| barium oxide | sodium formate molar mass | 153.326 g/mol | 68.007 g/mol phase | solid (at STP) | solid (at STP) melting point | 1920 °C | 260.5 °C density | 5.72 g/cm^3 | 1.919 g/cm^3
Units

Thermodynamic properties
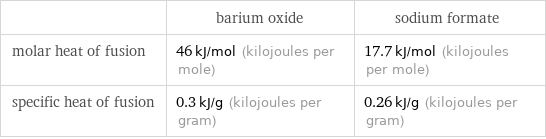
| barium oxide | sodium formate molar heat of fusion | 46 kJ/mol (kilojoules per mole) | 17.7 kJ/mol (kilojoules per mole) specific heat of fusion | 0.3 kJ/g (kilojoules per gram) | 0.26 kJ/g (kilojoules per gram)