Input interpretation

polar aprotic solvents | relative molecular mass
Summary
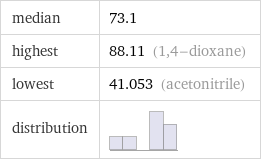
median | 73.1 highest | 88.11 (1, 4-dioxane) lowest | 41.053 (acetonitrile) distribution |
Ranked values
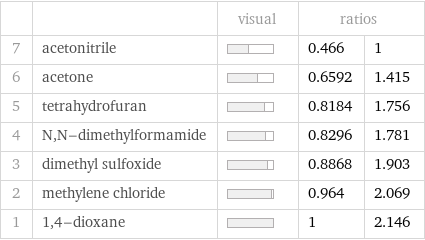
| | visual | ratios | 7 | acetonitrile | | 0.466 | 1 6 | acetone | | 0.6592 | 1.415 5 | tetrahydrofuran | | 0.8184 | 1.756 4 | N, N-dimethylformamide | | 0.8296 | 1.781 3 | dimethyl sulfoxide | | 0.8868 | 1.903 2 | methylene chloride | | 0.964 | 2.069 1 | 1, 4-dioxane | | 1 | 2.146
Relative molecular mass rankings
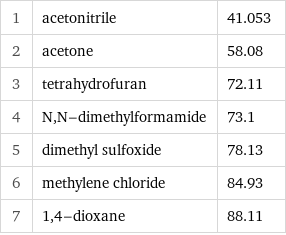
1 | acetonitrile | 41.053 2 | acetone | 58.08 3 | tetrahydrofuran | 72.11 4 | N, N-dimethylformamide | 73.1 5 | dimethyl sulfoxide | 78.13 6 | methylene chloride | 84.93 7 | 1, 4-dioxane | 88.11
Comparisons for median relative molecular mass 73.1

≈ ( 0.1 ≈ 1/10 ) × relative molecular mass of fullerene ( ≈ 721 )

≈ 0.38 × relative molecular mass of caffeine ( ≈ 194 )
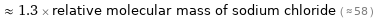
≈ 1.3 × relative molecular mass of sodium chloride ( ≈ 58 )
Corresponding quantities
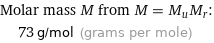
Molar mass M from M = M_uM_r: | 73 g/mol (grams per mole)

Molecular mass m from m = M_rM_u/N_A: | 1.2×10^-22 grams | 1.2×10^-25 kg (kilograms)